For all tests requested please order on Trak/GP ICE and use a single request form per specimen test request, detailing relevant clinical history.
Package each request separately in an individual specimen bag with 1 request form per specimen
All microbiology investigations are performed using advice/guidelines stated in UK Standards for Microbiology Investigations (SMIs): a comprehensive referenced collection of recommended algorithms and procedures for clinical microbiology. This is supplemented with best practice as recommended by the Scottish Microbiology and Virology Network.
All antimicrobial susceptibility testing is performed using guidelines and criteria as set by the European Committee on Antimicrobial Susceptibility Testing - EUCAST. Susceptibility testing is also supplemented with Clinical Laboratory Standards Institute (CLSI) criteria where appropriate.
Click on the name of the test to find out more.
ACTINOMYCETES CULTURE
Tested for in IUCDs where infection is suspected, and in other specimens if requested and if clinical details suggest it is appropriate. As prolonged culture is required for Actinomycetes a negative result will not be issued until the sample has been incubated for 10 days.
ASCITIC FLUID
Ensure aseptic technique while taking the sample. Blood culture bottles can be used but must also be accompanied by a portion of the sample in a sterile container if a cell count is required. Cell count and Gram film are reported on LIMS and are available in real time.
BLOOD CULTURES
The department uses the automated "BacT/ALERT Virtuo" system, in which the specimen is monitored continuously. Blood culture bottles should be available on the ward. Please check expiry date of bottles prior to using. Blood cultures are indicated not only for pyrexia but also for any clinical indication where a serious infection is suspected.
Please contact the department for blood culture sample bottles if they are not and arrange porters to uplift using option 2 on PorterTrak.
- Aseptic technique should be used as skin contaminants often confuse the clinical picture
- The injection area should be swabbed with Alcohol and allowed to evaporate dry
- 10 ml of blood should be injected into each bottle (anaerobic then aerobic) to give optimum test sensitivity
- For neonates take up to 4ml to a single BacTAlert Paediatric bottle
- With the exception of paediatric samples, bottles filled with less than 8ml will have a report issued stating ‘inadequately filled bottle – please interpret negative results with caution’
- Please do not remove barcodes from the blood culture bottles
- Always sample from a peripheral vein if possible. In cases where a CVP line infection is suspected blood cultures should be taken from both the peripheral vein and the line
Direct draw inoculating procedure
NOTE: If inoculating more than one type of BacT/ALERT blood culture bottle using a butterfly collection set and direct draw adapter cap, inoculate first the aerobic bottle (blue) and then the anaerobic bottle (pink/purple) so that any oxygen trapped in the tubing will not be transferred to the anaerobic bottle.
NOTE: Monitor the direct draw process closely at all times to assure proper flow is obtained and to avoid flow of the bottle contents into the adaptor tubing. Due to the presence of chemical additives in the culture bottle, it is important to prevent the possible backflow and subsequent adverse reactions by following the steps below.
- Hold culture bottle upright below patient’s arm.
- Collect the blood using a butterfly collection set and the BacT/ALERT Blood Collection Adapter Cap directly into the culture bottle
- Release the tourniquet as soon as blood starts to flow into the culture bottle, or within 2 minutes of application.
- Do not allow the culture bottle contents to touch the stopper or the end of the needle during the collection procedure. A contaminated culture bottle could contain positive pressure, and if used for direct draw, may cause reflux into the patient’s vein.
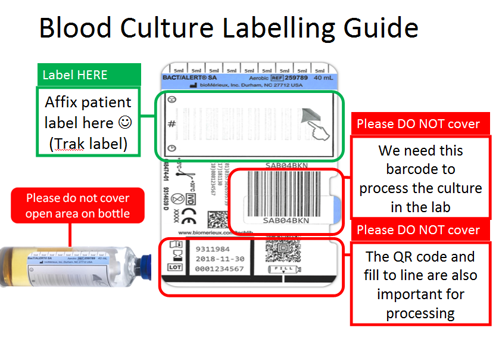
Blood culture bottles should be labelled as follows:
BONE MARROW
The department stores and issues collection bottles used for Mycobacterium investigation, the laboratory should be contacted to request bottles prior to sampling. Microbiology will then send the sample direct to the Regional Mycobacterium Reference Laboratory for testing.
CAPD FLUID
Fluid from CAPD bags may be sent for microbiological investigation if peritonitis is suspected. Send the fluid in a sterile container (at least 50mls). Please do not send the whole C.A.P.D. bag.
CEREBRO-SPINAL FLUID
- Use aseptic technique
- For routine microbiology culture 1-3 ml of fluid in a sterile universal container is required (5 to 10mls for TB investigation)
- More may be needed if extra tests are requested. Do not refrigerate
- Send immediately to the laboratory (PTS is recommended) as an urgent sample and warn the laboratory to expect it.
- The results of cell count and Gram film examinations are reported on LIMS and are available in real time
- Note that spectrophotometry for Xanthochromia is not performed in the Microbiology department this is a Biochemistry request
- If the patient is immunocompromised, further tests can be discussed with the clinical Microbiologist
MRSA Screening
- For MRSA screening samples include Nasal & Perineum (Throat if the patient refuses perineum) refer to the NHS GG&C MRSA policy for sampling information
Click on disease specific information and then select Methicillin Resistant Staphylococcus Aureus (MRSA) from the NHSGGC Infection Prevention and Control Guidelines webpage.
A duo eSwab which covers nose & perineum in one specimen container is used however single liquid eSwabs are available to use for single sites (e.g. throat).
PECOS Codes:
Duo eSwab: 4E047S02
Single eSwab: 90CE.A
Please note these come with a short expiry date. Please check expiry date on swab before use. Expired swabs will not be processed.
Examples:
- Nose, throat & perineum screening
- Duo eSwab for nose and perineum – Trak order as MRSA Screen, Duo swab - Nose-perineum
- Single eSwab for throat – Trak order as MRSA Screen, site throat
- Nose & perineum screening
- Duo eSwab for nose and perineum – Trak order as MRSA Screen, Duo swab - Nose-perineum
- Nose & throat screening
- Single eSwab for nose - Trak order as MRSA Screen, site nose
- Single eSwab for throat – Trak order as MRSA Screen, site throat
CPE/CRO SCREENING, MSSA, RENAL VRE
- For CPE/CRO screening please send Rectal swab or faeces. Refer to the NHS GG&C CPE toolkit
- For Bulk Renal MSSA screening please contact the lab in advance to discuss requests and allow stock to be ordered to perform the test
- For Renal VRE screening send a Rectal swab
EYE SWABS
Conjunctival discharge or pus is sampled using a plain swab in transport medium.
Please contact the laboratory in advance of sampling if you require culture of corneal scrapings and/or Acanthamoeba culture.
If Chlamydia or viral infection is suspected, contact the Virology lab at GRI for advice.
FAECES
Collect in a sterile container (25ml) with or without spoon. Please do not contaminate the outside of the container with faeces. Badly contaminated specimens will not be accepted.
Faecal samples are cultured selectively for species of Salmonella, Shigella, Campylobacter and for E. coli O157.
Consider and request faecal parasites if there is a history of foreign travel (always include details of travel history when requesting), HIV infection or if the diarrhoea is chronic.
All faeces specimens are routinely examined for Cryptosporidium.
Diarrhoeal stool samples (samples that conform to the shape of the container) are routinely screened for C. difficile toxin on patients >3 years but please state if this is clinically suspected. It is accepted that if a patient’s diarrhoea is intermittent, or if they are incontinent of faeces, it may not be possible to obtain a diarrhoeal sample. In this case the clinical details on the request form should make this fact clear, and the requesting clinician should highlight that they nonetheless wish C. difficile toxin testing to be performed on the sample. There is no requirement to test for clearance in known toxin-positive patients and re-testing in cases of suspected relapses/re-infection should not be performed within 10 days of the original positive sample.
Fresh samples (<2hrs old) are required. If unable to deliver samples within 2 hours then samples should be refrigerated. Storage at ambient temperature is not recommended. Specimen may be passed into a clean, dry, disposable bedpan or similar container and transferred into a CE marked leak proof container. The specimen is unsatisfactory if any residual soap, detergent or disinfectant remains in the pan.
Diarrhoeal outbreaks should be discussed with both the Infection Control nurses and the clinical Microbiologist.
GASTRIC BIOPSIES (for Helicobacter pylori culture)
Gastric biopsy samples for Helicobacter pylori are processed at Glasgow Royal Infirmary – Small biopsies should be in a sterile container with a small amount of saline (<2ml) to prevent drying. Do NOT place in formalin fixative. These samples should be referred to the laboratory urgently for Helicobacter culture and the laboratory contacted.
GENITAL TRACT SWABS
High and Low Vaginal Swabs are taken using a plain swab, which is then inserted into charcoal Amies transport medium. There is no need to send both swabs, HVS is the preferred sample type. Urethral swabs should be taken with a special small diameter urethral swab.
JOINT ASPIRATES
Ensure aseptic technique when collecting the specimen. Send in a sterile container (25ml). Gram films are routinely carried out on these specimens and the result of the Gram film will be reported on LIMS in real time.
The Microbiology department does not look for crystals in joint aspirates.
MYCOLOGY
Mycology samples for superficial infection are processed at Glasgow Royal Infirmary – mycology specimens should be collected in either sterile universal containers or relevant mycology collection pack. Microscopy and or culture will be performed depending on sample type and clinical history.
Galactomannan antigen testing is performed on clotted blood or on bronchoalveolar lavage/bronchal washings.
Cryptococcal antigen testing is performed on CSF samples and serum as requested.
PLEURAL/PERICARDIAL FLUID
Ensure aseptic technique while taking the sample. Send in a sterile container (25ml). The results of a Gram film will be reported in telepath in real time.
Please state if TB is suspected and where possible send an additional sample requesting AAFB & TB culture.
SPUTUM
Sputum is collected in a sterile container (25ml or 60ml).
Sputum bacteriology is often of doubtful value due to inadequate sampling of the lower respiratory tract and inevitable contamination with salivary flora. Results are greatly improved following collection of a supervised, deeply coughed specimen. The physiotherapist may be useful in obtaining a good quality specimen, or in obtaining induced sputum where the patient is unable to expectorate.
Please state on the form if T.B. is suspected and request T.B.culture and AAFB.
Routine AAFB microscopy will not be performed unless TB is requested. Three early morning samples of deeply coughed sputum should be sent for this investigation. Do NOT send via the pneumatic tube system.
Broncho-alveolar lavage fluid (BAL) is routinely cultured for common respiratory pathogens and TB; Legionella and fungal cultures are performed on request. Please state on the request form if any other investigations are required along with a relevant clinical history.
THROAT SWABS
Sample the inflamed area with a plain swab with Amies transport medium. Please clearly indicate if there is a relevant travel history.
TIPS
Tips from removed intra-vascular lines can be sent for culture. Please send in a sterile container.
Culture of urinary catheter tips is thought to be of little diagnostic value and these sample types are not accepted for testing.
TISSUE
Samples of tissue (usually from theatre) should be sent in a sterile container.
Any positive Gram stains will be telephoned to the ward. Please state if there is a patient history of I.V. drug injection as these specimens are processed differently.
URINARY TRACT SAMPLES
Mid-stream and catheter specimen of urine:
- Fill a sterile primary urine container containing boric acid (red cap).
- Request culture and sensitivity (C&S)
- For catheter specimens the sample is best taken immediately after the catheter has been inserted
- If the catheter has been in situ for a while the specimen should ideally be taken from the tube of the catheter with a syringe and needle, not from the catheter bag
Volume Rejection Criteria
- GP Adult -Small volume specimens (< 8ml) are rejected
- Hospital Adult -Small volume specimens (< 5ml) are rejected
- Paediatrics -Small volume specimens (< 1ml) are rejected
As many CSU cultures represent colonisation rather than infection, antibiotic sensitivities are not routinely issued.
Indications for Microscopy
Microscopy will be performed in situations where the value of microscopy is supported by evidence based guidelines. These are:
- Children under 3 years of age
- Children aged 3 or over - NICE guidelines state that if leucocyte esterase is positive and nitrite is negative then arrange with the Microbiology laboratory for urine microscopy and culture to be performed. Otherwise all specimens from children (>=3) will be cultured only
- Patients with glomerulonephritis
- Specimens from patients who are post renal transplant
- Suprapubic Aspirate (SPA)
Early morning sample of urine/EMU for TB:
- Take first thing in the morning by completely emptying the bladder into special EMU containers (150 ml)
- Take samples on three consecutive mornings
- Do NOT send via the pneumatic tube system
WOUND/SKIN INFECTION
Sample the deeper layers of a wound or ulcer site using a plain swab in Amies transport medium. Gram stains are not routinely carried out on swab specimens.
Pus sample is preferred if available, collect in a sterile container. Gram stains are routinely performed on these samples.
Wound swabs are processed for aerobic and anaerobic organisms. Contact the laboratory for antibiotic advice if required.
Skin scrapings from the advancing edge of a suspected fungal lesion should be sent in the relevant mycology collection pack.
WATER / ENVIRONMENTAL SAMPLING
GRI has an Environmental Laboratory that carries out air sampling, environmental testing and water testing.
Water testing for indicator organisms can only be performed by prior arrangement with the laboratory.
Particle counts & settle plates are performed by prior arrangement with the laboratory.
Contact the Environmental Laboratory direct on: 0141 956 0443
AD HOC ENVIRONMENTAL MONITORING
Any request for environmental monitoring should be through discussion and arrangement with the Infection Control Consultant and Laboratory Senior Management. This includes theatres or any other sites under the remit of GG&C.
The infection control consultant will arrange the testing requirements directly with the laboratory after completion of the environmental testing request form.