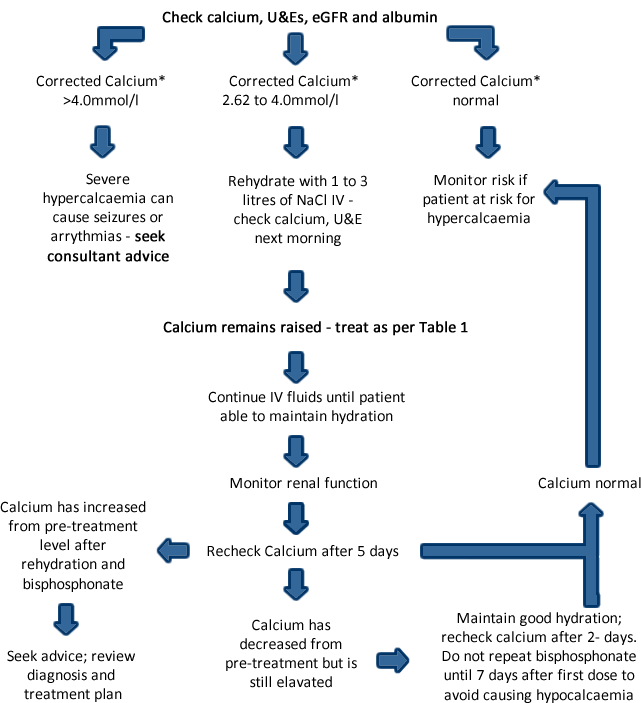
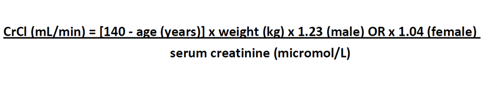- If the patient is asymptomatic with corrected calcium* between 2.62 mmol/l and <2.8 mmol/l, rehydrate with fluids and review as per table in treatment section.
- Explain signs, symptoms and treatment options to the patient, family and carers.
- Not all symptoms resolve after treatment. This may be due to other cause(s) or underlying disseminated disease.
- Bisphosphonates may cause mild flu-like symptoms.
- Bisphosphonates are implicated risk factors in osteonecrosis of the jaw, osteonecrosis of the auditory canal and atypical fractures.
- Where possible, patients should have regular dental checks and avoid invasive dental procedures whilst on treatment.
- The severity of symptoms is related to the rate of increase; not the level of corrected calcium.
- The speed of recurrence may signify a poor prognosis.
- Review current treatments for underlying disease.
- Untreated severe hypercalcaemia can be fatal.
*Corrected calcium = Measured calcium +0.022 x (40 - serum albumin g/l)
Corrected calcium
Tables are best viewed in landscape mode on mobile devices
Table 1
| Corrected calcium* (mmol/l) |
Drug and Dose |
Diluent and maximum infusion rate |
| |
Disodium pamidronate |
|
| 2.62 to 3.0 |
15mg to 30mg |
500ml NaCl 0.9% over > 60 minutes |
| 3.0 to 3.5 |
60mg |
500ml NaCl 0.9% over > 60 minutes |
| 3.5 to 4.0 |
90mg |
500ml NaCl 0.9% over > 90 minutes |
| >4.0 |
90mg |
500ml NaCl 0.9% over > 90 minutes |
| |
Zoledronic acid |
|
| >3.00 |
4mg |
100ml NaCl 0.9% over 15 minutes |
If corrected calcium >3.0mmol/l, some units routinely give pamidronate 90mg as a higher dose.
*Corrected calcium = Measured calcium +0.022 x (40 - serum albumin g/l)
Reduced doses in renal impairment
- Disodium pamidronate in renal impairment, seek advice.
- eGFR >30ml/min: Minimum infusion period 90 minutes, maximum infusion rate 20mg/hour; consider dose reduction.
- eGFR <30ml/min: avoid except in life threatening hypercalcaemia where specialist advice should be sought to determine if benefit outweighs risk.
Zoledronic acid in renal impairment
- Patients with tumour induced hypercalcaemia (TIH) and deteriorating renal function should be appropriately assessed to determine if the potential benefit of treatment with zoledronic acid outweighs the possible risk.
- After 24-48 hours of rehydration, consider a single IV dose of zoledronic acid 4mg in 100ml sodium chloride 0.9% over ≥ 15 minutes. Dose alteration may not be needed in mild to moderate renal impairment in patients with TIH (ie eGFR >30ml/min).
- Avoid if eGFR <30ml/min, refer to Summary of Product Characteristics (SPCs) (www.medicines.org.uk) for further details.