For the purposes of reporting CNS tumours, the NICE guidance on Improving outcomes for people with brain and other CNS tumours defines a neuropathologist as “an accredited pathologist who is registered as a neuropathologist or histopathologist, has specialist expertise in neuro- oncology, and takes part in the national External Quality Assurance (EQA) scheme for neuropathology organised by the British Neuropathological Society”. NICE guidelines also emphasise the central role of the MDT meeting in the management of CNS tumours.Pathologists reporting CNS tumours should attend these meetings and participate in the relevant EQA scheme.
Pathology

This consensus document is not a rigid constraint on clinical practice, but a concept of good practice against which the needs of the individual patient should be considered. It therefore remains the responsibility of the individual clinician to interpret the application of these guidelines, taking into account local service constraints and the needs and wishes of the patient. It is not intended that these consensus documents are applied as rigid clinical protocols.
Principles
- To provide a standard approach to the diagnosis of adult glioma across Scotland, using WHO Classification of Tumours of the Central Nervous System4
- To work to RCPath Standards and datasets for reporting cancers: Dataset for histopathological reporting of tumours of the central nervous system in adults, including the pituitary gland1
- To deliver histological diagnosis initially, with a final integrated molecular pathology report within 21 days of surgery
Clinical details, as provided by the submitting clinician on the request form, should be recorded on the pathology report. Relevant clinical history is essential to provide adequate interpretation of the histological findings. We recommend following ICCR Tumours of the Central Nervous System Histological Assessment Reporting Guide3 this includes location and focality of the tumour, neuroimaging findings, previous diagnoses and therapies.
This should include dimensions of the specimens provided, and detail any intra-operative diagnosis offered at the time of surgery. If possible, a fresh frozen sample should be retained for molecular diagnostics.
This should include a detailed microscopic description of the tumour including WHO CNS Grade4.
Figure 1 shows an algorithm applied in the diagnosis of an adult glioma, and this should be undertaken for all cases as required. Additional molecular diagnostics, such as methylation profiling, RNA fusion panels, and DNA WGS, should be available for cases as required.
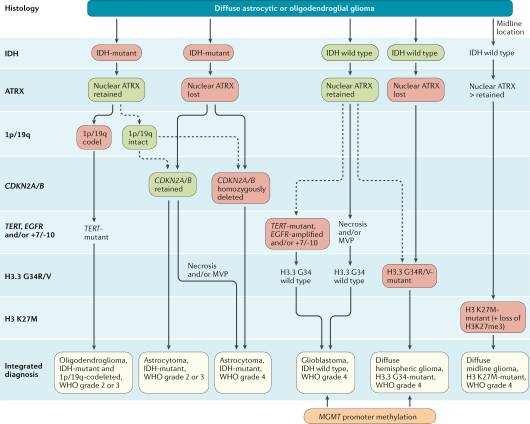
Figure 1: Diagnostic algorithm for glioma diagnosis5.
A final integrated report incorporating histopathological diagnosis and molecular diagnosis should be issued, in line with WHO Classification of Tumours of the Central Nervous System 4.
A neuropathologist should present all cases at the MDT.
All departments issuing integrated neuropathology reports should show evidence of internal audit in relation to diagnostics, and regular audit activities to review compliance with published standards. All consultants must undertake relevant CPD, and successfully complete appraisal annually.
- Royal College of Pathologists. Standards and datasets for reporting cancers: dataset for histopathological reporting of tumours of the central nervous system in adults, including the pituitary gland. 2020.
- Public Health Scotland (PHS). Data Definitions for the National Minimum Core Dataset to support the introduction of Brain/CNS Quality Performance Indicators. 2021.
- International Collaboration on Cancer Reporting (ICCR). Tumours of the Central Nervous System. 2018.
- National Institute for Health and Clinical Excellence (NICE): Guidance on Cancer Services Improving Outcomes for People with Brain and Other CNS Tumours; The Manual.2006
- WHO Classification of Tumours Editorial Board. Central Nervous System Tumours: WHO Classification of Tumours. Volume 6. 5th Edition. Lyon: IARC; 2021.
- Weller M, et al . EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021 Mar;18(3):170-186.
