Administration of Adrenaline (Epinephrine) 1:1000 injection to any patient suffering from anaphylaxis

Protocol for the administration of Adrenaline (Epinephrine) 1:1000 Injection or autoinjector device without a prescription for a named individual by registered health professionals employed by NHS Borders or in NHS Borders GP Practices
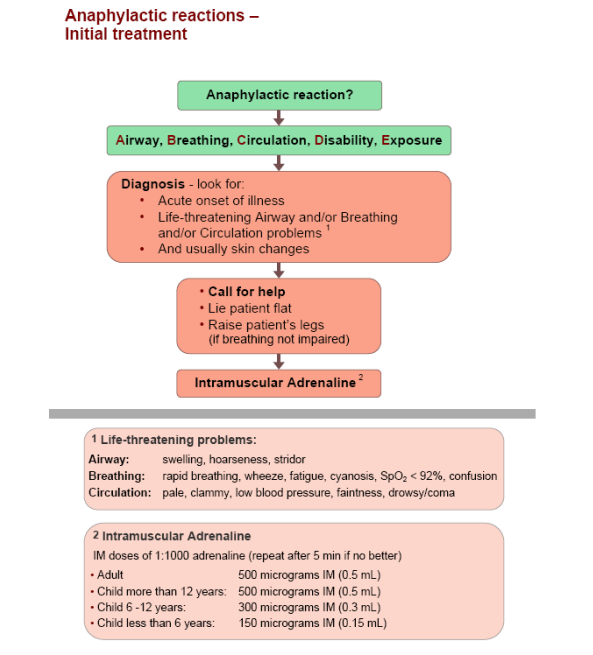
TREATMENT OF ANAPHYLAXIS IN ALL CASES OF SUSPECTED ANAPHYLAXIS DIAL 999
IMMEDIATELY OR 2222 IF IN THE BGH
1. This Protocol relates to the following specific preparation:
| Name of medicine, strength, formulation |
Adrenaline (Epinephrine) injection |
| Legal status | POM Prescription Only Medicine |
| Storage |
Not to be kept in fridge |
| Dose |
IM doses of 1:1000 adrenaline (repeat after 5 min if no better) Adult: 500 micrograms IM Child more than 12 yrs: 500 micrograms IM Child 6-12 yrs: 300 micrograms IM Child less than 6 yrs: 150 micrograms IM 1:10,000 strength of adrenaline to be excluded under this protocol |
| Route/method | Intramuscular injection |
| Frequency | Repeat dosing if required after 5 minutes up to a maximum of 3 doses over fifteen minutes |
| Total dose Quantity (Maximum) |
3 |
| Advice to Patients | Reassure patient, explain procedure and course of action. Monitor patient and provide full handover to supporting staff or paramedics |
| Relevant Warnings |
Possible Side Effects:
|
|
Staff should also record in patients notes cause of anaphylaxis and this information should be disseminated widely to ensure future exposure to |
|
| Follow up Arrangements |
Initiate CPR, Ensure patient transferred to hospital if in the community. Ensure patient is reviewed by medical staff |
2. Clinical condition:
| Clinical Condition to be treated |
Emergency management of acute anaphylaxis/ anaphylactoid allergic reaction |
| Criteria for inclusion |
Do not administer adrenaline on the basis of an isolated skin rash. |
| Criteria for exclusion | None |
| Action if excluded | Not relevant |
| Action if declines | Ring 999 (Community) or 2222 (In the BGH) and transfer to medical care urgently |
| Interactions with other medicaments |
Although some medications interact, timing is absolutely critical and is unlikely to allow a full medication history. Risks of not administering outweigh the risk of giving in this life-saving situation. |
3. Records: Patient Notes, MIU Records, GP system, dependent on location
The following records should be kept (either paper or computer based):
- The GP practice, clinic, hospital, and ward or department.
- The patient name and CHI number.
- The medicine name, dose, route, time of dose(s), and where appropriate, start date, number of doses and or period of time, for which the medicine is to be supplied or administered.
- Drug batch number and expiry.
- The signature and printed name of the registered healthcare professional that supplied or administered the medicine.
- Explanation that the Adrenaline was given following this protocol.
- Whether patient met the inclusion criteria and whether the exclusion criteria were assessed.
- Quantity supplied / received and current stock balance.
Preparation, audit trail, data collection and reconciliation
- Stock balances should be reconcilable with receipts, administration, records and disposals on a patient by patient basis.
Storage
- Injection: Do not store above 25°C. Keep container in outer carton.
- Auto-Injector device: Keep in the outer carton. Do not store above 25 °C
Not to be kept in fridge.
4. Professional Responsibility:
- All registered Health Professionals will ensure he/she has the relevant training and is competent in all aspects of medication, including contra-indications and the recognition and treatment of adverse effects. He/she will attend training updates as appropriate. For those involved in immunization, regular anaphylaxis updates are mandatory.
- Nurses will have due regard for the NMC Code of Professional Conduct, standards for conduct, performance and ethics (2008) and NMC Standards for Medicines Management (2008).
5. Sources of Evidence used for the PGD creation :
- www.BNF.org
- Resuscitation Council (UK) Anaphylaxis guidance
- Resuscitation Council (UK) Anaphylactic Treatment Algorithm
- Summary of Product Characteristics: Epipen www.medicines.org.uk
Resuscitation Council (UK) Anaphylactic Reactions Initial Treatment