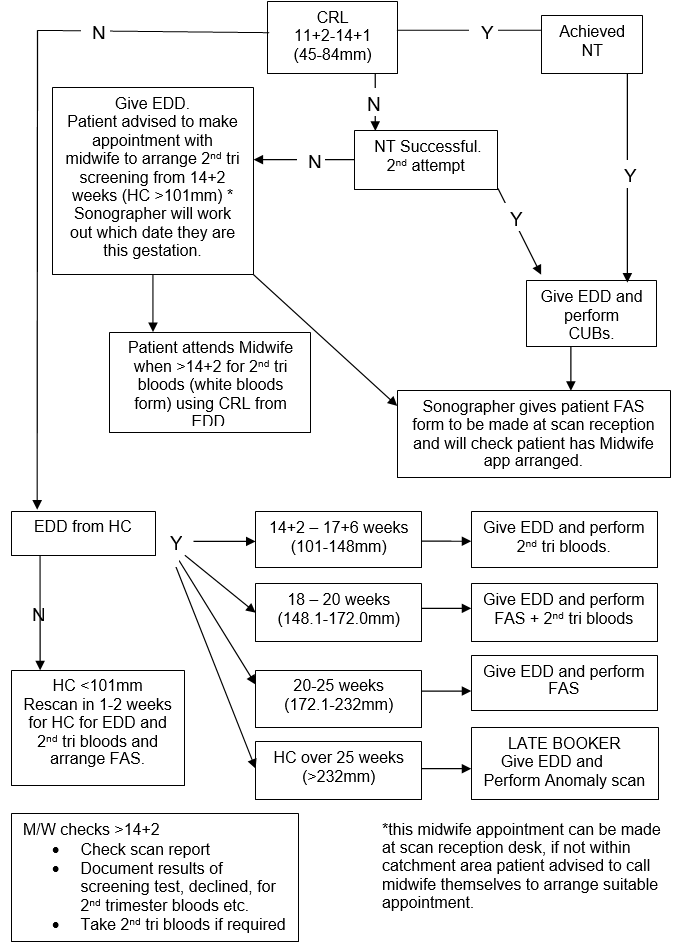
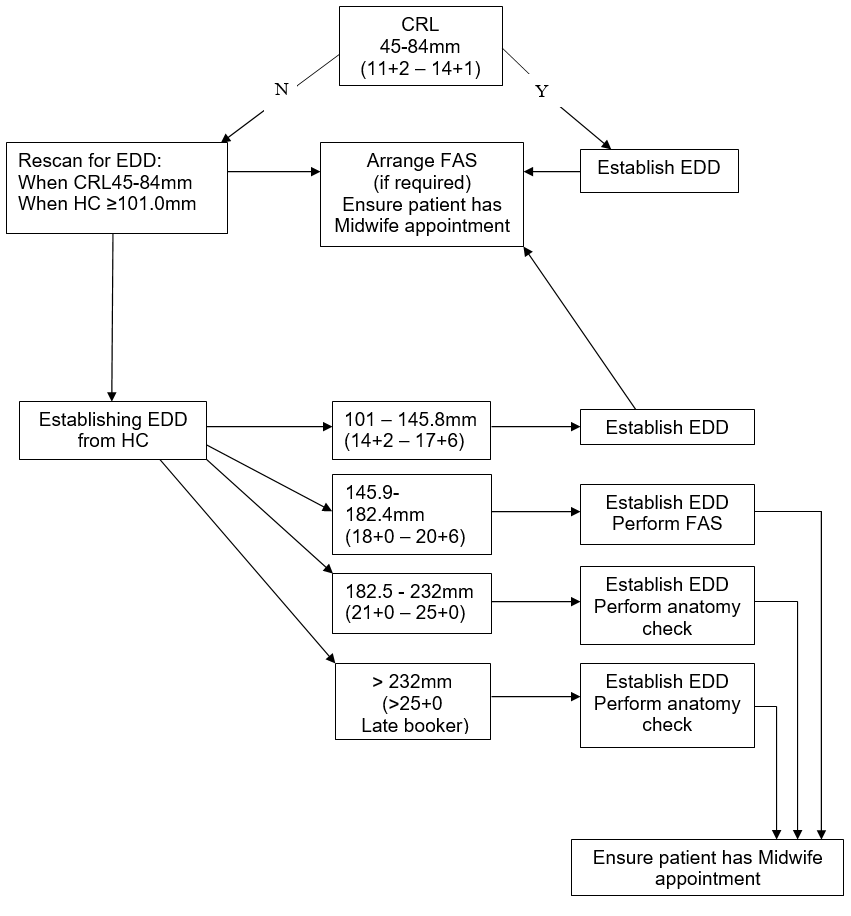
The HC should be calculated using the recommended values of Altman and Chitty as per BMUS recommendations.
Technique for calculation of HC:
A cross-sectional view of the fetal head at the level of the ventricles should be obtained. The image should have the midline echo lying as close as possible to the horizontal plane.
The following landmarks should be identified and the image frozen:
- rugby ball shape;
- centrally positioned, continuous midline echo broken at one third of its length by the cavum septum pellucidum;
- anterior walls of the lateral ventricles centrally placed around the midline;
- the choroid plexus should be visible within the posterior horn of the ventricle in the distal hemisphere.
- Callipers should be placed on the outer border of the occipital and frontal bones as close as possible to the midline across the longest part of the skull.
If HC measurements cannot be made then EDD should be calculated using the femur length (FL)
Technique for calculation of FL:
The image should be obtained with the femur lying as close as possible to the horizontal plane. The full length of the bone should be visualised with soft tissue visible at both ends. Calipers should be placed at the centre of the ‘U’-shape at each end of the bone.