- ISTH has definitions for standardizing bleeding symptoms which may be helpful in assessing patients. Please note that these definitions were primarily developed for the purpose of retrospectively classifying bleeding events e.g. for the purpose of clinical trials. Clinical judgement is required when assessing patients who are bleeding.
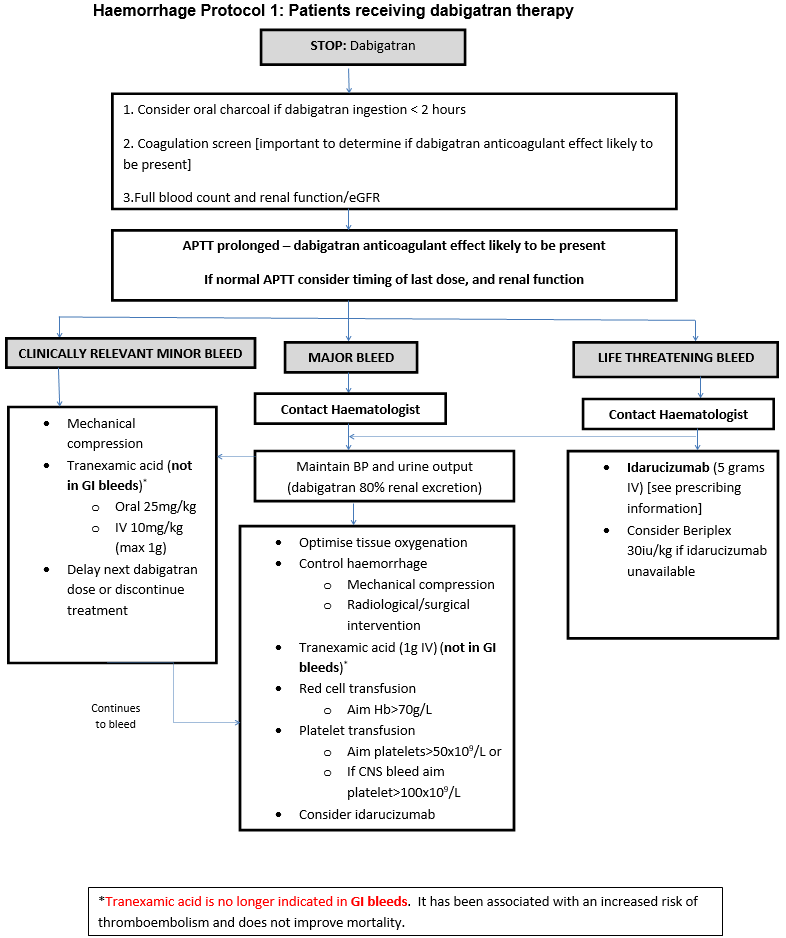
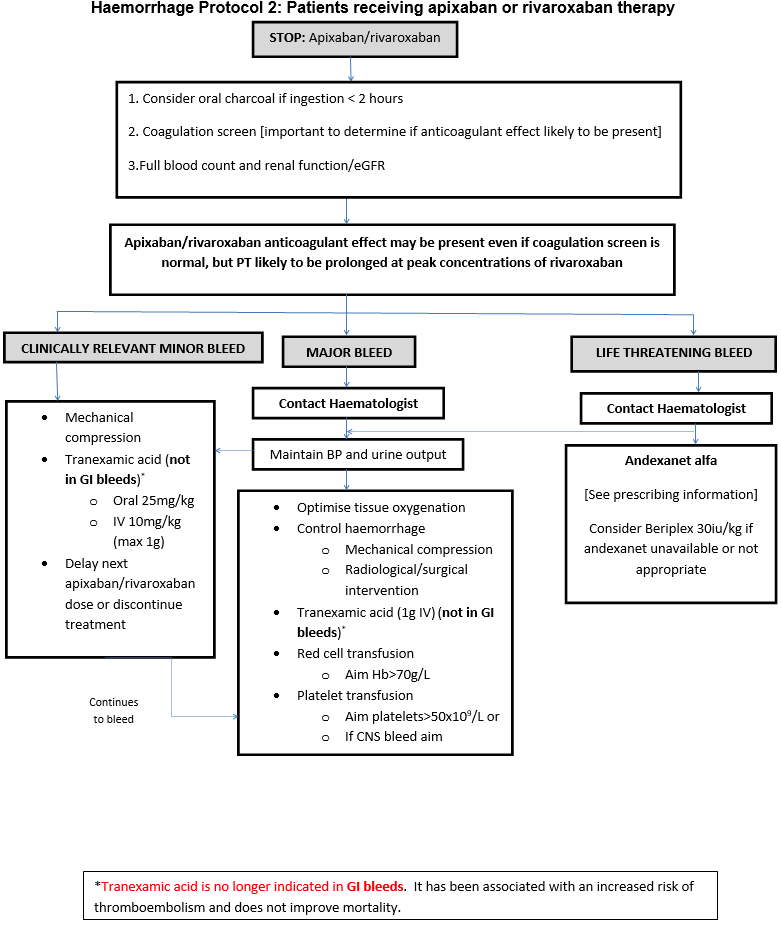
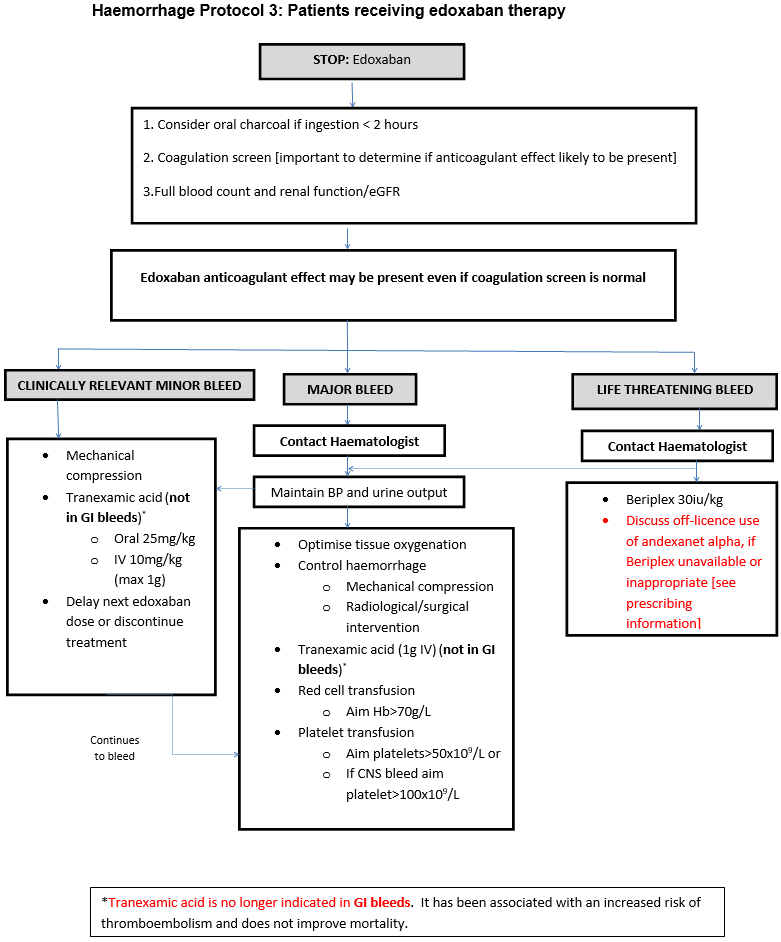
Major bleeding in Non-Surgical Patients
- Symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra-articular or pericardial, or intramuscular with compartment syndrome.
- Bleeding causing a fall in haemoglobin of 2g/dL or more, or leading to transfusion of two or more units of whole blood or red cells.
Major bleeding in Surgical Patients
- Bleeding that is symptomatic and occurs in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, pericardial, in a non-operated joint, or intramuscular with compartment syndrome, assessed in consultation with the surgeon.
And/or
- Extrasurgical site bleeding causing a fall in haemoglobin level of 2 g/dL or more, or leading to transfusion of two or more units of whole blood or red cells, with temporal association within 24–48 h to the bleeding.
And/or
- Surgical site bleeding that requires a second intervention (open, arthroscopic, endovascular) or a haemarthrosis of sufficient size as to interfere with rehabilitation by delaying mobilization or delayed wound healing, resulting in prolonged hospitalization or a deep wound infection.
And/or
- Surgical site bleeding that is unexpected and prolonged and/ or sufficiently large to cause hemodynamic instability, as assessed by the surgeon. There should be an associate fall in haemoglobin level of at least 2 g/d, or transfusion, indicated by the bleeding, of at least two units of whole blood or red cells, with temporal association within 24 h to the bleeding.
Clinically relevant minor bleeding
Does NOT meet the criteria for major bleeding above but does prompt a clinical response and leads to one of the following:
- A hospital admission for bleeding
- A physician guided medical or surgical intervention for bleeding
- A change in antithrombotic therapy, including interruption or discontinuation of anticoagulant therapy