Integrated Patient Pathway for Chronic Sialorrhea in Adults (Guidelines)

Drooling is the unintentional loss of saliva from the mouth. In the adult population it can be associated with neurological disorders such as Parkinson’s disease, motor neurone disease (MND) and stroke. Contrary to popular belief, drooling is rarely caused by hypersalivation but is more often related to neuromuscular and/or sensory dysfunction in the oral stage of the swallow1.
Complication
Drooling in the adult patient has various repercussions, ranging from physical difficulties such as dehydration, foul oral odour, perioral skin maceration and increased risk of aspiration pneumonia, to social ramifications such as embarrassment, isolation and increased dependency. As such, drooling can have a negative effect on quality of life, so much so that many patients rate drooling as their worst symptom1.
Sialorrhea can cause: choking, skin problems, infections, distress and embarrassment for people with neurological conditions including: motor neuron disease (MND), Parkinson’s disease, multiple sclerosis, Huntington’s disease, stroke, cerebral palsy and spinocerebellar ataxias. It can make it difficult to be understood when speaking; it can make eating and drinking harder; and it can cause people to withdraw from social activities and even employment, triggering social isolation and financial hardship15. Unmanaged drooling also has a very significant impact on unpaid carers, causing emotional distress, social isolation, and increased domestic tasks15.
Sialorrhea is often untreated, as existing treatment options are not clinically appropriate for some people with neurological disorders. The medicines which are available may cause side effects such as dry mouth and skin irritation and they may make common cognitive and neuropsychiatric symptoms worse in patients with Parkinson’s disease or sequel of head injury. Sialorrhea is often managed using practical aids (such as bibs) and interventions from speech and language therapists. However, many people with neurological conditions find it difficult to access speech therapy to aid with saliva and swallowing.
More recently, clostridium botulinum neurotoxin type A (Xeomin®) has been accepted for use within NHS Scotland and this fact offer an opportunity to treat sialorrhea and to review the existing drooling pathways. (Scottish Medicines Consortium: SMC2; Indication under review: for the symptomatic treatment of chronic sialorrhea due to neurological disorders in adults). Clostridium botulinum neurotoxin type A improved unstimulated saliva flow rate and the Global Impression of Change Scale compared with placebo2.
Medical Evaluation |
Social Evaluation |
Oromotor Assessment |
|
|
|
First line treatment is usually non-pharmacological, for example: bibs, speech and language therapy, and occupational therapy. However, some symptoms may be severe enough to require pharmacological therapy.
Identification of the associated risks, specific to the patient:
(Medical):
- Posterior drooling > increased risk of aspiration > increased risk of progressive lung diseases and recurrent infection
- Anterior drooling > presence of saliva on the cheek > frequent wiping > increased risk of skin irritation and breakdown
(Psychosocial):
- Anterior drooling > excessive saliva on clothes/ face > changing of clothes – may damage materials in front of them. Embarrassment – can affect the young and old. Additional burden upon the carer.
Thin or watery saliva?
Thin: This watery type of saliva, is more likely to be observed spilled outside onto the mouth. In MND patients, around two or three pints of excessive saliva is a commonly reported symptom3.
Thick: Thickened mucus within the mouth and throat, producing swallowing difficulties. Phlegm present in the airways may be difficult to cough up, due to weakened muscles or an ineffective cough4. This type of saliva can build up at the back of the throat, due to dehydration, mouth-breathing or open mouth posture. This may result in the partial blockade of airways. Consider the use of mucolytics.
The symptoms should be assessed using the drooling and severity scale:
| Severity | Frequency | Total | |||||||
| 1 Never Lips dry |
2 Mild Only lips |
3 Moderate Lips and chin |
4 Severe Onto clothing |
5 Profuse Onto objects |
1 None |
2 Occasionally |
3 Frequently | 4 Constant | |
If the score is 5 or less then symptoms are not severe enough for the risk of treatment to outweigh the benefit5.
The Drooling Severity and Frequency Scale (DSFS) has two dimensions: drooling severity and drooling frequency.
- Drooling severity is rated on a scale of 1 (dry) to 5 (profuse drooling).
- Drooling frequency is rated on a scale of 1 (never) to 4 (constantly).
- The total DSFS score can therefore range from 2 to 916.
Create goals of treatment (e.g.):
- Improve control of oromotor secretion
- Enhance ability to behaviourally manage secretions
- Reduction of saliva or rerouting of salivary flow
With this, identify the practical methods of reaching these goals. What would the patient be most comfortable with (regarding the associated side effects of treatment) and to what standard would the patient accept the excessive salivary challenges?
Following this, the most appropriate treatment strategies should be chosen. Patients with chronic and progressive neurological conditions usually require a multidisciplinary team approach, to ensure the care is consistent and tailored towards the patient’s needs. This will also ensure all the patient’s concerns are addressed, including the needs of their family’s and caregivers.
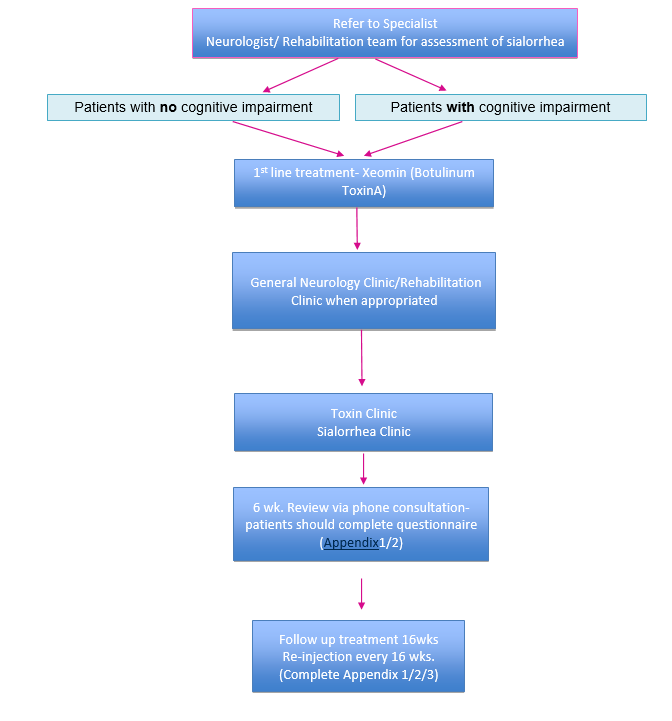
In November 2019 the SMC approved2 Xeomin, botulinum toxin type A (also called: incobotulinumtoxinA) (see in pharmacological treatments) as the only clostridium botulinum neurotoxin type A for treating chronic sialorrhea in adults caused by neurological conditions.
Clinical experts consulted by the SMC considered that the place in therapy of clostridium botulinum neurotoxin type A is to provide a licensed treatment option for patients with chronic sialorrhea due to neurological disorders. Specialist training is required for administration of a medicine into the salivary glands: this may have service implications.
NICE TA 605 recommended Xeomin (incobotulinumtoxinA) as an option for treating chronic sialorrhea in adults caused by neurological conditions6.
NICE have recommended Xeomin as both a first- and second-line treatment option:
The company (Merz UK) positioned Xeomin (its preparation of botulinum neurotoxin type A) as both a first- and second-line treatment option. The committee therefore considered it as:
- an alternative first-line treatment to non-pharmacological management such as bibs, speech and language therapy and occupational therapy (referred to as standard care by the company) and to anticholinergics and
- as an alternative second-line treatment to standard of care (in line with the 3 NICE guidelines)6.
When choosing the first line of treatment, it is important to understand the realistic goals the patient wants to achieve, and how soon they would like to be attained.
Non-pharmacological methods - in combination with alternative therapy:
Patients can be referred to specialists, such as occupational therapists, physiotherapists, dietitians and speech and language therapists, who may be able to provide advice on the below points.
Note that these methods are not recommended for those with posterior drooling or with thick secretions.
Patients can be referred to specialists, such as occupational therapists, physiotherapists, dietitians and speech and language therapists, who may be able to provide advice on the below points.
Note that these methods are not recommended for those with posterior drooling or with thick secretions.
- Optimize conditions – Identify if the patient is taking any other medication, which might increase drooling (cholinergics / anti-epileptics) – consider the effect of these treatments / the effect of taking them away or reducing the dosage. Discuss the effects with the patient, ensure they are receiving their most desired outcome (least burden). Don’t want further side effects requiring polypharmacy (producing an additional burden). Dietary changes, like avoiding alcohol and citrus fruits may be recommended7.
- Oromotor and orosensory strategies – active and passive exercises and sensor applications. Widely used by clinicians – but no agreement of their effectiveness. Time consuming, but no associated adverse effects.
- Behavioural strategies – The encouragement of swallowing/ mouth wiping/ improving head control. Provision of advice e.g. Discourage open mouth and thumb sucking. Prompting and reinforcement, to make it a learnt behaviour. No associated adverse events. These strategies can be effective up to a point. It may be difficult/ not feasible if an older/ less compliant patient (likely with progressive neurological disease) – more likely to be forgetful/ less able to pick up new behaviours. Positive outcomes largely reported. This wouldn’t be practical for patients with severe motor limitations.
- Oral appliance – Prosthetic device to improve jaw stability, lip closure and tongue mobility – to modify behaviour. For example, a suction unit may be loaned out, so the patient can manage salivary secretions from their own home. May be impractical or uncomfortable. Low level of evidence that these are effective.
Pharmacological treatments
- The recommended treatments act via antimuscarinic innervation. Prescribers will therefore need to review the appropriateness of pre-existing medication for other symptoms.
- Doses should be titrated upwards to the accepted level of dryness/ presence of side effects, or until a maximum dose is reached
a) Botulinum Neurotoxin Type A
The Botulinum neurotoxin acts as a neuroglandular blocking agent, inhibiting the release of acetylcholine, reducing the salivary production and therefore reducing sialorrhea6.
Xeomin (Merz Pharmaceuticals) is the only botulinum neurotoxin licensed for sialorrhea treatment. Intended for chronic sialorrhea due to neurological disorders in adults. 100 units per treatment session, 30 units to parotid gland per side and 20 units to submandibular gland per side6.
This treatment method has been seen to improve the severity and frequency of sialorrhea in patients in multiple placebo-controlled studies and real-world studies. The SIAXI trial being the largest, prospective, randomized, double-blind, placebo controlled multicentre study investigating the use of Xeomin, with the results proving significant reductions in unstimulated salivary flow rate 4 weeks post treatment, compared to a placebo8.
b) Anti-cholinergic drugs
If the above methods are inadequate, next in line are the anti-cholinergic agents. These act to inhibit the action of acetylcholine at muscarinic receptors, reducing the production of saliva. Success response rates vary widely, however limited evidence is available in adult patients.
Different medications come under the bracket of an anticholinergic:
Oral tablet/ solution to be taken 2 to 3 times per day. Risks with elderly. Side effects: dry mouth, drowsiness, blurred vision, tachycardia, arrhythmias, flushing, constipation, urine retention, vomiting, nasal congestion, headache, decreased sweating, dizziness, pupillary dilation and mental 9,10,11.
Anticholinergic medications are associated with more severe side effects, particularly with cognitive risks seen in PD patients, because of blocking muscarinic receptors in the brain. A low dose should initially be administered, and then titrated up until the effect is deemed beneficial towards the quality of life for the patient12.
- Glycopyrronium bromide (licensed to treat sialorrhea in children) – Usually an oral tablet, but an oral suspension is available which can be given orally or via a feeding tube. A tablet has to be taken 2 to 3 times a day, risks in those with neurological conditions (amnesiac). An approximate annual cost of £986 to £9864. A subcutaneous injection can also be administered, when needed. Glycopyrronium- Sialanar17,18,19 is licensed for use in children and recommended by NICE/SMC17,18. Off Label for Adults.
- Hyoscine butylbromide – Oral tablet, which can also be crushed and dissolved in water. Subcutaneous form can also be administered.
- Hyoscine hydrobromide – Comes in patch form, which is applied behind the ear and is changed every 72 hours. Tablets or subcutaneous injection also an option.
- Atropine sulfate - An eye-drop, administered sublingually 3 to 6 times a day. Benefits are short lasting. Should not be administered directly from original container, avoiding possible overdose. Dose should be given using a disposable dropper or placed onto a spoon first.
- Tricyclic antidepressants – Low dose given at night, orally or via feeding tube. Can cause sedation.
Invasive treatments
Invasive options are often considered when the excessive drooling remains profuse and persistent despite maximal behavioural therapy and pharmacological treatment. The side effects associated with the pharmacological methods may also lead to a patient’s preference for surgery. As these surgical methods and irreversible, they are often reserved for the last resort of treatment. This is where it is important to categorize the patient’s sialorrhea as being transitory or chronic. Only those patients with severe chronic sialorrhea, not responding to pharmacological interventions, should consider this type of treatments:
- Duct removal or ligation (submandibular, parotid or both) – longer lasting, however risk factors need to be considered
- Relocation/ rerouting of salivary gland ducts.
- Interruption of the parasympathetic supply to the glands.
- Intraductal laser photocoagulation of the bilateral parotid ducts (less invasive)
- Radiation therapy – rare treatment, usually reserved for elderly patients who cannot undergo or tolerate drug therapies. Seen to be an effective treatment regarding the effect upon patient burden13 and in ALS patients14 lasts up to 6 months.
Side effects are usually minimal, but xerostomia, wound infection (duct ligation/ removal), malignancy and mucositis (radiation therapy) are possibilities.
Medication review and monitoring
(Include patient self-assessment Appendix 1/2/3)
A follow-up appointment of the most recent treatment performed. Looking at the short and long term effects. Weekly self assessment by patient/carer to be discussed with the clinician at the six week follow up: the patient (or caregiver) should collect information every week whilst on treatment, considering the sialorrhea symptoms, associated side effects and effects upon quality of life for the patient and care-giver.
The symptoms should be assessed using the drooling severity and frequency scale as mentioned above (Appendix 2). If one treatment does not produce an improvement in the scale after 4 cycles on the highest tolerance, the next treatment in line should be tried. Adverse events should be monitored regularly.
Conservative management
This comes alongside medication review. The need to visit specialists regarding behavioural management may change over time when changes in symptoms are observed or side effects appear or wain.
Further treatment
If the ongoing treatment is proving to be successful from the patient and care-giver’s point of view, then continue with this treatment, remaining within the guidelines of the treatment to ensure safety. New symptoms may become apparent with the treatments, which may require further treatments. Dry mouth, for example, which can be treated using Corsodyl, Difflam or artificial saliva. The presence of thick secretions may also be treated with mucolytics or additional simple conservative measures.
Patients should be assessed to fill out self-assessment Form (Appendix 1) and at the 16 weeks follow up Appendix 2 and 3 should be completed.
To be completed by the patient - click here
To be completed by the patient - click here
To Be completed by the HCP - click here
[1] therapeutic-guidelines/palliative-care/saliva-management-sialorrhoea/
[3] Young CA. Treatment for sialorrhea (excessive saliva) in people with motor neuron disease/ amyotrophic lateral sclerosis. The Cochrane database of systemic reviews. 2011; (5).
[4] Rafiq MK. Respiratory management of motor neuron disease a review of current practice and new developments. Practical Neurology. 2012; 12(3):166-76;
[5] Rashnoo, P., Daniel, S. Drooling quantification: Correlation of different techniques. International Journal of Pediatric Otorhinolaryngology. 2015:79;8,1201-1205
[6] NICE xeomin (botulinum neurotoxin type A for treating chronic sialorrhoea
[7] Bavikatte G, Sit PL and Hassoon A. Management of drooling of saliva. Br J Med Pract 2012; 5: 25-31
[8] Jost WH, Friedman A, Michel O. SIAXI: placebo-controlled, randomized, double-blind study of incobotulinumtoxinA. Neurology 2019; 92: e1982.
[9] Banfi P, Ticozzi N, Lax A. A review of options for treating sialorrhoea in amyotrophic lateral sclerosis. Respir Care 2015; 60: 446-454
[10] Pellegrini A, Lunetta C, Ferrarese C, Sialorrhoea: how to manage a frequent complication of motor neuron disease
[11] Martindale Pharma. Glycopyrronium bromide 200 micrograms/ ml solution for injection.
[12]Richardson et al. Anticholinergic drugs and risk of dementia: case-control study. BMJ 2018;360
[13] Weikamp JG, Schinagl DA, Verstappen CC. Botulinum toxin-A injections vs radiotherapy for drooling in ALS.
[14] Neppelberg E, Haugen, DF, Thorsen L, Radiotherapy reduces sialorrhoea in amyotrophic lateral sclerosis. Eur J Neurol2007; 14: 1373-1377
[15] https://www.mndscotland.org.uk/latest/news/new-treatment-for-excessive-drooling-in-mnd/
[16] Rashnoo P, Daniel SJ. Droolingquantification:correlation of different techniques. Int J PediatrOtorhinolaryngol2015;79:1201–5.
[17] NICE severe sialorrhoea guideline
[19] https://www.ema.europa.eu/en/documents/product-information/sialanar-epar-product-information