The British Association for Psychopharmacology (BAP 2017) grades their recommendations (A-D) with (A) having the strongest evidence base. Table 1 summarizes these
Table 1: BAP 2017 guidelines recommendations
Type of dementia | Evidence to support |
Alzheimer’s disease | AChE inhibitors for cognition in mild to severe Alzheimer’s disease (A) |
Lewy bodies and Parkinson’s disease dementia | Rivastigmine and donepezil for the treatment of dementia with lewy bodies and Parkinson’s disease dementia (A) |
Vascular dementia | There is type I evidence showing small cognitive improvements with both AChE inhibitors and memantine in vascular dementia. However, benefits in terms of global outcome are not seen and adverse events for AChE inhibitors (but not memantine) are significantly greater than placebo. Evidence indicates that neither AChE inhibitors nor memantine should be prescribed to people with vascular dementia, though those with mixed vascular dementia and Alzheimer’s disease may benefit (A) |
Frontotemporal dementia | AChE inhibitors are not recommended for the treatment of frontotemporal dementia (A) |
Mild cognitive impairment | There is type I evidence that AChE inhibitors are not effective in reducing the risk of developing Alzheimer’s disease (A) |
National Institute of Clinical Evidence (NICE NG97 2018) recommends:
- The three AChE inhibitors as monotherapies are recommended as options for managing mild to moderate Alzheimer's disease
- Memantine as monotherapy is recommended as an option for managing Alzheimer's disease for people with:
- Moderate Alzheimer’s disease who are intolerant or have a contraindication to AChE inhibitors
- Severe Alzheimer’s disease
- For people with an established diagnosis of Alzheimer's disease who are already taking an AChE inhibitor:
- consider memantine in addition to an AChE inhibitor if they have moderate disease
- offer memantine in addition to an AChE inhibitor if they have severe disease.
- Pharmacological management of non-Alzheimer's dementia
- Offer donepezil or rivastigmine to people with mild to moderate dementia with Lewy bodies.
- Only consider galantamine for people with mild to moderate dementia with Lewy bodies if donepezil and rivastigmine are not tolerated.
- Consider donepezil or rivastigmine for people with severe dementia with Lewy bodies
- Consider memantine for people with dementia with Lewy bodies if AChE inhibitors are not tolerated or are contraindicated.
- Only consider AChE inhibitors or memantine for people with vascular dementia if they have suspected co-morbid Alzheimer's disease, Parkinson's disease dementia or dementia with Lewy bodies.
- Do not offer AChE inhibitors or memantine to people with frontotemporal dementia.
- Do not offer AChE inhibitors or memantine to people with cognitive impairment caused by multiple sclerosis.
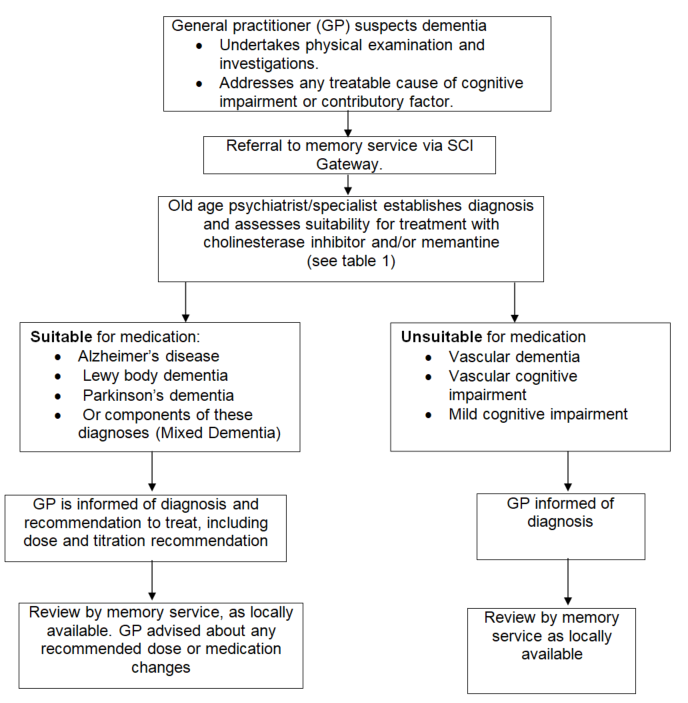
Both BAP and NICE guidelines recommend input from specialists for diagnosis and ongoing monitoring and outline a clear role for General Practitioners (GP) in prescribing and ongoing care.