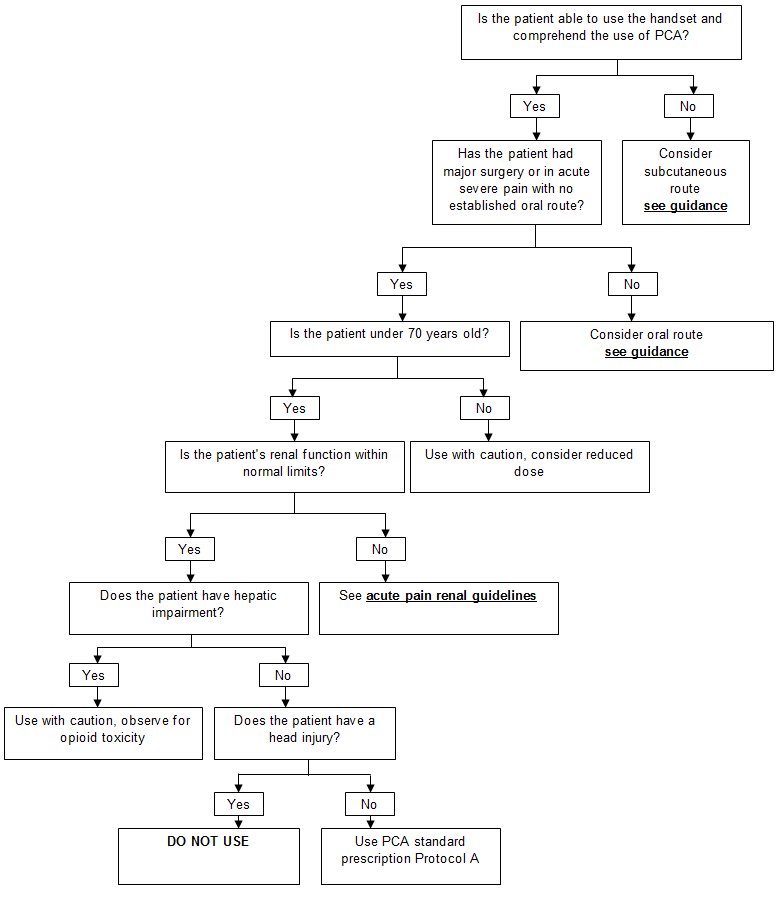
The standard prescription is Protocol A
- Morphine
- 1mg bolus
- 5 minute lock out
The prescriber must complete a PCA chart, including the following details :
- Patient identification
- Date
- Operation/reason for PCA
- Type of pump and pump number
- Naloxone prescription must be signed for on the PCA
- Prescribe PCA on HEPMA
If a ward doctor or non-medical prescriber wishes to prescribe an alternative dose, discussion should first take place with the Acute Pain Nurse or an Anaesthetist.
Background infusions should only be nursed in critical care
The Prescription should be prescribed in the regular or 'As required' section on HEPMA and affix a “PCA/Epidural Opioid in Progress” onto HEPMA.
Alternatives to Morphine may be used if required, please contact Acute Pain Nurse or ITU anaesthetist.
Anti-emetics must be prescribed on HEPMA if patient is to have PCA, see TAM postoperative nausea and vomiting guidelines.
Access and lines
- Peripheral cannula will be used most commonly but PCA can also be administered via central line.
- PCA can be administered on its own but is preferably administered with maintenance fluids to maintain line patency.
- The syringe must be connected to the cannula using a PCA administration set, which contains an anti-syphon valve to prevent free flow and anti-reflux valve if other fluids are connected to the same venflon.
- Vygon Protect-A-Line extension set must be used without maintenance fluids and Vygon Protect-A-Line extension set must be used with maintenance fluids.
PCA Pumps
- All PCA Infusion must be delivered via the Agilia PCA pump
- Agilia PCA pumps are located in the equipment library