Wound management guidelines and formulary (Formularies)

What's new / Latest updates
08/07/24 - ActiForm Cool hydrogel 10cm x 10cm no longer used - replaced with 10cm x 15cm.
The NHSH Wound Management Guidelines & Formulary has had a refresh and update due to the award of the National Procurement contract for Wound Management Products. This was done by the Wound Management Formulary Product Subgroup which had a wide range of specialty input. The awarded products were reviewed Nationally, and a group of Tissue viability Specialists worked to creating a more unified National list of products where the majority of items were agreed by all Health Boards. We have also ensured that both A&B and North Highland have unified to one formulary list of products. Making this available on TAM has also provided more support to independent care homes so patient treatment can be more aligned across NHSH.
For HHSCP and A&BHSCP
The Wound Management Guidelines and Formulary has been broken down into sections that are accessible by clicking on the section index on the left hand side of the screen. Each section covers a topic that has been created by the specialists in that area to ensure up-to-date guidance with links to the intranet and internet sites. All the information provided has been agreed by the NHSH Wound Formulary Group.
Introduction
NHS Highland Wound Management Guidelines & Formulary have been developed by the Wound Management Formulary Products Subgroup created by the Tissue Viability Leadership Group.
The aim of this document is to provide practitioners with guidance on both wound care and appropriate product selection. First line dressings should be used for the initial management of all wounds unless there is a clinical reason documented in the patient’s medical notes describing why this would be unsuitable.
This formulary can also be used as an educational tool to promote cost effective prescribing in the management of wounds across the NHS Highland Area.
Medical Representative Advice
In order to reinforce the appropriate use and management of dressings and promote formulary compliance, medical representatives must not leave wound product samples in clinical areas. NHS Highland staff should not accept such product samples and if evaluation and trial are required, contact Wound Management Formulary Products Subgroup.
Wound Formulary Group Members
- Ali Cattanach – Chairperson
- Elaine Farrell – TVN
- Findlay Hickey - Pharmacist
- Claire MacKenzie – Podiatry
- Heather Johnston – ED
- Morven Jack – Vascular Nurse
- Denise Gilchrist – Pharmacist A&B
- Nancy MacKay - TVN
- Thomas Ross - Pharmacist
- Wendy Anderson – Formulary Assistant
- Louise Shakespeare – Lymphoedema
- Tracy Sutherland - Prescribing Support Nurse
- Jenny Munro - Advanced Practice Physio (Continence)
- Stacey Evans-Charles - TVN Lead Nurse
| Product | Supplier Code | Pack size | Pecos item sku code | Single item price (Pecos) | Single item price (Drug Tariff/ GP10) |
| GREEN: most COST-EFFECTIVE option RED: most EXPENSIVE option | |||||
| ALGINATE DRESSINGS | |||||
| ActivHeal Alginate 5cm x 5cm | 10007432 | 5 | 272143 | ||
| ActivHeal Alginate 10 cm x 10cm | 10007431 | 5 | 272150 | ||
| ActivHeal Alginate 2.5cm x 30cm | 10007428 | 5 | 251797 | ||
| Flaminal Forte 15 gram tube | 324 2963 | 5 | 138067 | ||
| Flaminal Hydro 15 gram tube | 324 2971 | 5 | 138074 | ||
| BARRIER PRODUCTS | |||||
| Barrier Cream for protection (no wounds or broken skin) - Cavilon Durable Barrier Cream 2g sachet | 3392GS | 20 | 241842 | ||
| Barrier Films (for superficial breaks with no signs of infection) - Cavilon No Sting Barrier Film 28ml pump | 23346E | 1 | 231041 | ||
| Foam applicator (for peri-wound protection during dressing changes) Protect Foam Applicator 1ml | 7265400 | 5 | 268153 | N/A | |
| Foam applicator (for peri-wound protection during dressing changes) Protect foam applicator 3ml | 7265402 | 5 | 268160 | N/A | |
| EMOLLIENT PRODUCTS | |||||
| Please follow link. | |||||
| DEBRIDEMENT PAD | |||||
| UCS Debridement | DT500 | 10 | 217106 | ||
| Wound Debridement pad with handle - Debrisoft | 33224 | 5 | 216451 | ||
| Wound Debridement pad Debrisoft 10cm x 10cm 3432 | 3432 | 10 | 230266 | ||
| FIBROUS HYDROCOLLOID DRESSINGS | |||||
| Aquacel Extra 5 x 5cm | 420671 | 10 | 184057 | ||
| Aquacel Extra 10 x 10cm | 420672 | 10 | 184064 | ||
| Aquacel Extra 15 x 15cm | 420673 | 5 | 184071 | ||
| Aquacel Extra ribbon 2 x 45cm | S7503 | 5 | 031719 | ||
| Restricted to use in Theatres and for post-operative wound management only | |||||
| Aquacel Surgical 9 x 15cm (ORTHOPAEDIC USE ONLY) | 412018 | 10 | 203352 | N/A | |
| Aquacel Surgical 9 x 15cm (ORTHOPAEDIC USE ONLY) | 412019 | 10 | 200269 | N/A | |
| Leukomed Sorbact 10cm x 25cm (FOR C-SECTION ONLY) | 7619904 | 20 | Direct from Supplier | ||
| Leuokmed Sorbact 10cm x 30cm (FOR C-SECTION ONLY) | 7619905 | 20 | Direct from Supplier | ||
| HIGH ABSORBENCY WOUND PADS - 1st line choice | |||||
| Kliniderm Super Absorbent 10cm x 10cm | 40511701 | 50 | 269617 | ||
| Kliniderm Super Absorbent 10cm x 20cm | 40511702 | 50 | 269624 | ||
| Kliniderm Super Absorbent 20cm x 30cm | 40511704 | 10 | 269631 | ||
| Kliniderm Super Absorbent 20cm x 40cm | 40511705 | 10 | 269648 | ||
| HIGH ABSORBENCY WOUND PADS - 2nd line choice | |||||
| Cutimed Sorbion Sachet XL 25cm x 45cm | 7324001 | 5 | 264926 | ||
| Cutimed Sorbion Sachet S 10cm x 10cm | 7323206 | 10 | 264889 | ||
| Cutimed Sorbion Sachet S 20cm x 30cm | 7323218 | 10 | 264919 | ||
| HIGH ABSORBENCY WOUND PADS - 3rd line choice - lymphorrea or heavy exudate management ONLY | |||||
| ConvaMax Superabsorber Non-adhesive 10cm x 10cm | 422567 | 10 | 272051 | ||
| ConvaMax Superabsorber Non-adhesive 15cm x 20cm | 422571 | 10 | 272068 | ||
| ConvaMax Superabsorber Non-adhesive 20cm x 30cm | 422573 | 10 | 271139 | ||
| ConvaMax Superabsorber Non-adhesive 20cm x 40cm | 422574 | 10 | 271146 | ||
| HYDROCOLLOID DRESSINGS | |||||
| Duoderm X thin 4.3 x 3.8cm | S001SP | 10 | 173761 | N/A | |
| Duoderm X thin 10 x 10cm | S161 | 10 | 031658 | ||
| Duoderm X thin 5 x 10cm | S163 | 10 | 034857 | ||
| Duoderm X thin 15 x 15cm | S162 | 10 | 031665 | ||
| Duoderm X thin 9 x 25cm | S172 | 10 | 165452 | ||
| Duoderm X thin 9 x 35cm | S173 | 10 | 165469 | ||
| HYDROGEL DRESSINGS | |||||
| ActiForm Cool hydrogel 10cm x 15cm | 88302 | 5 | 093205 | ||
| ActiForm Cool hydrogel 5cm x 6.5cm | 88300 | 5 | 269662 | ||
| Activheal Hydrogel Amorphous (with preservatives) 15g | 10007419 | 10 | 134074 | ||
| POLYURETHANE FOAM FILM DRESSINGS (WITH BORDER) | |||||
| Kliniderm foam border 7.5 x 7.5cm | 40514828 | 5 | 216659 | ||
| Kliniderm foam border 10 x 10cm | 40514829 | 5 | 216666 | ||
| Kliniderm Foam border 15 x 20cm | 40514834 | 5 | 216703 | ||
| POLYURETHANE FOAM FILM DRESSINGS (NO BORDER) | |||||
| Kliniderm foam silicone no border 5 x 5cm | 40514823 | 5 | 216598 | N/A | |
| Kliniderm foam silicone no border 10 x 10cm | 40514824 | 5 | 216604 | N/A | |
| Kliniderm foam silicone no border 15 x 15cm | 40514825 | 5 | 216628 | ||
| POLYURETHANE FOAM FILM HEEL DRESSINGS - no shaped dressings to be used | |||||
| Kliniderm foam silicone border 10 x 10cm | 40514829 | 5 | 216666 | ||
| PRIMARY WOUND CONTACT LAYER | |||||
| Atrauman 5 x 5cm | 499550 | 50 | 134021 | ||
| Atrauman 7.5 x 10cm | 499553 | 50 | 230501 | ||
| Atrauman 10 x 20cm | 499536 | 30 | 052301 | ||
| PRIMARY SILICONE WOUND CONTACT LAYER | |||||
| Kliniderm Silicone Wound Contact Layer 7.5cm x 10cm | 40514881 | 10 | 267774 | ||
| Kliniderm Silicone Wound Contact Layer 12cm x 15cm | 40514885 | 10 | 267781 | ||
| Kliniderm Silicone wound contact layer 20cm x 30cm | 40514883 | 5 | 267798 | ||
| SELF ADHERENT DRESSINGS (NON WATERPROOF) | |||||
| 365 Non Woven Island 5 x 7.2cm | 50 | 214150 | |||
| 365 Non Woven Island 10 x 10cm | 50 | 214167 | |||
| 365 Non Woven Island 10 x 15cm | 50 | 214174 | |||
| 365 Non Woven Island 10 x 20cm | 25 | 214181 | |||
| 365 Non Woven Island 10 x 25cm | 25 | 214198 | |||
| 366 Non Woven Island 10 x 35cm | 15 | 238460 | |||
| SELF ADHERENT DRESSINGS (WATERPROOF) | |||||
| 365 Transparent Island 5 x 7.2cm | 50 | 217168 | |||
| 365 Transparent Island 8.5 x 9.5cm | 50 | 217045 | |||
| 365 Transparent Island 8.5 x 15.5cm | 50 | 217052 | |||
| 365 Transparent Island 10 x 25cm | 50 | 217076 | |||
| 365 Transparent Island 20 x 10cm | 50 | 217069 | |||
| 365 Transparent Island 30 x 10cm | 50 | 217083 | |||
| Restricted to use in Theatres and for post-operative wound management only | |||||
| Tegaderm + Pad 5 x 7cm | 50 | 140497 | |||
| Tegaderm + Pad 9 x 10cm | 25 | 117826 | |||
| Tegaderm + Pad 9 x 15cm | 25 | 117840 | |||
| Tegaderm + Pad 9 x 20cm | 25 | 117857 | |||
| Tegaderm + Pad 9 x 25cm | 25 | 047451 | |||
| Tegaderm + Pad 9 x 35cm | 25 | 117819 | |||
| VAPOUR PERMEABLE ADHESIVE FILM DRESSINGS | |||||
| 365 Film 6 x 7cm | 100 | 170944 | |||
| 365 Film 10 x 12cm | 50 | 170951 | |||
| 365 Film 15 x 20cm | 30 | 170968 | |||
| Restricted to use in Theatres and for post-operative wound management only | |||||
| Tegaderm Film 6 x 7cm | 100 | 219773 | |||
| Tegaderm Film 10 x 12cm | 50 | 219780 | |||
| Tegaderm Film 15 x 20cm | 10 | 219797 | |||
| Dressing Pack | |||||
| Procedure Pack Universal with Medium Glove | 36510017/M | 90 | 236312 | ||
| Specialist Products | |||||
| BIOSYNTHETIC CELLULOSE FIBRE DRESSING WITH PHMB - contact TVN for advice & guidance | |||||
| Suprasorb X+ PHMB 14 x 20cm | 20542 | 5 | 274017 | ||
| CHARCOAL DRESSINGS - 1st line | |||||
| Clinisorb 10 x 10cm | 2305 | 10 | 024728 | ||
| Clinisorb 10 x 20cm | 2310 | 10 | 024735 | ||
| Clinisorb 10 x 25cm | 2315 | 10 | 173389 | ||
| CHARCOAL DRESSINGS - 2nd line for specialist use - contact TVN or Podiatry for advice and guidance | |||||
| CarboFlex 10cm x 10cm | S7660 | 10 | 117277 | ||
| CarboFlex 15cm x 20cm | S7662 | 10 | 034871 | ||
| DAAC TECHNOLOGY | |||||
| Cutimed Sorbact swab coated 4 x 6cm | 72164-01 | 5 | 178988 | ||
| Cutimed Sorbact swab coated 7 x 9cm | 72165-01 | 5 | 178995 | ||
| Cutimed Sorbact swab coated ribbon 2 x 50cm | 72166-00 | 20 | 178964 | ||
| HONEY DRESSINGS - Antimicrobial | |||||
| No Tulle honey dressings - use L-Mesitran ointment with Atrauman | |||||
| L-Mesitran Soft 15g | 104.01 | 14 | 269952 | ||
| L-Mesitran Ointment 20g | 203.01 | 14 | 268023 | ||
| HYDROGEL AMORPHOUS WITH SURFACTANT | |||||
| Prontosan Irrigation Solution 350ml | 400403 | 10 | 133895 | ||
| Prontosan Gel X 50g | 203.01 | 1 | 193585 | ||
| Prontosan Irrigation Solution 1000ml - RESTRICTED TO HOSPITAL USE ONLY | 400446 | 10 | 235568 | N/A | |
| IODINE – CADEXOMER IODINE - Antimicrobial | |||||
| Iodoflex Paste 4 x 6cm, 5g | 66001301 | 5 | 037452 | ||
| Iodoflex Paste 8 x 6cm, 10g | 66001302 | 3 | 071333 | ||
| Iodoflex Paste 8 x 10cm, 17g | IOD129M | 2 | 071340 | ||
| IODINE-POVIDONE IODINE - Antimicrobial | |||||
| Inadine 5 x 5cm | P01481 | 25 | 039654 | ||
| Inadine 9.5 x 9.5cm | P01491 | 10 | 039661 | ||
| LARVAE THERAPY (Bio Bag) - Contact TVN for advice and guidance | |||||
| MATRIX MODULATING DRESSING - only used with agreement from Podiatry or TVN | |||||
| Promogran Prisma with silver 28 x 28cm (antimicrobial) Protease modulator | PS2028.S | 10 | 117697 | ||
| Promogran Prisma with silver 123 x 123cm (antimicrobial) Protease modulator | PS2123 | 10 | 128976 | ||
| UrgoSTART Contact 5 x 7cm Protease inhibitor | 550276 | 10 | 216772 | ||
| UrgoSTART Contact 10 x 10cm Protease inhibitor | 550278 | 10 | 216789 | ||
| UrgoSTART plus Pad 6 x 6cm Protease inhibitor | 552301 | 10 | 246755 | ||
| UrgoSTART plus Pad 10 x 10cm Protease inhibitor | 552302 | 10 | 246762 | ||
| POLYURETHANE FOAM FILM DRESSING with 3D FIT TECHNOLOGY | |||||
| Biatain Silicone 10 x 10cm | 3445 | 10 | 271238 | ||
| POLYURETHANE FOAM DRESSING WITH SURFACTANT | |||||
| Polymem Non-adhesive 10 x 10cm | 5044 | 60 | 242290 | N/A | |
| POLYURETHANE FOAM SILICONE - NO BORDER (FOR PODIATRY USE ONLY) | |||||
| Mepilex XT 10 x 11cm | 211160 | 5 | 200993 | ||
| SILVER DRESSINGS - Antimicrobial | |||||
| Aquacel AG + Extra 10 x 10cm | 413567 | 10 | 201488 | ||
| Aquacel AG + Extra 15 x 15cm | 413568 | 5 | 201495 | ||
| Aquacel AG + Extra 5 x 5cm | 413566 | 10 | 201471 | ||
| Aquacel AG + Extra 2 x 45cm | 413571 | 5 | 215575 | ||
| Flamazine (silver sulfadiazine) 1% cream 50g tube - Contact TVN for advice and guidance | Pharmacy only | ||||
The NHS Highland Wound Management Guidelines and Formulary (WMG&F) has been developed by theTissue Viability Leadership Group, a multidisciplinary group of professionals.
The aim of the WMG&F is to provide practitioners with guidance and a selection of products based oneffectiveness, suitability, acceptability and cost-effectiveness. Practitioners should use a formulary productwhere at all possible. In the vast majority of cases, formulary products are both on the Scottish NationalProcurement Contract (which controls products purchased by Scottish Hospitals) and the Scottish DrugTariff (which states what can be prescribed on NHS prescriptions and GP stock orders in primary caresettings).
NOTE: The product sizes listed are those available on PECOS. Additional sizes may be available onprimary care prescription.
Prices have been taken from PECOS, the Scottish Drug Tariff or MIMS (www.mims.co.uk) and are correct as of May 2018 but are subject to change.
Soft wadding synthetic
These are used under bandaging and plaster of Paris, for absorbency of exudate, protecting the area from pressure and distributing pressure more evenly.
|
Product |
Pack size |
Pecos item code |
Single item price (Pecos) |
Single item price (Drug Tariff) |
|
Soft wadding synthetic (Soffban not on Drug Tariff – alternative K-Soft one size only) |
||||
|
Soffban 5cm x 2.75m |
1 |
170357 |
BEST PRICE |
Not on Drug Tariff |
|
Soffban 7.55cm x 2.75m |
1 |
170364 |
BEST PRICE |
Not on Drug Tariff |
|
Soffban10cm x 2.75m |
1 |
170371 |
BEST PRICE |
Tariff size: 10cm x 3.5m - 10cm x 4.5m |
|
Soffban 15cm x 2.75m |
1 |
170388 |
BEST PRICE |
Not on Drug Tariff |
|
Soffban 20cm x 2.75m |
1 |
170470 |
BEST PRICE |
Not on Drug Tariff |
Light support bandage crepeand light support knitted
These are used in the prevention of oedema; they are also used to provide support for mild sprains and joints, but their effectiveness has not been proven for this purpose. Since they have limited extensibility, they are able to provide light support without exerting undue pressure.
|
Product |
Pack size |
Pecos item code |
Single item price (Pecos) |
Single item price (Drug Tariff) |
|
Light Support Bandage Crepe – Premierband |
||||
|
Light Support Bandage Crepe 5cm x 4.5m |
1 |
170357 |
BEST PRICE |
|
|
Light Support Bandage Crepe 7cm x 4.5m |
1 |
170364 |
BEST PRICE |
Tariff size: 7.5cm x 4.5m |
|
Light Support Bandage Crepe 10cm x 4.5m |
1 |
170371 |
BEST PRICE |
|
|
Light Support Bandage Crepe 15cm x 4.5m |
1 |
170388 |
BEST PRICE |
|
|
Product |
Pack size |
Pecos item code |
Single item price (Pecos) |
Single item price (Drug Tariff) |
|
Light Support Knitted – Urgo K Lite |
||||
|
Light Support Bandage Knitted 5cm x 4.5m |
1 |
040223
|
BEST PRICE |
|
|
Light Support Bandage Knitted 7cm x 4.5m |
1 |
001330
|
BEST PRICE |
|
|
Light Support Bandage Knitted 10cm x 4.5m |
1 |
001323
|
BEST PRICE |
|
|
Light Support Bandage Knitted 15cm x 4.5m |
1 |
059331 |
BEST PRICE |
|
Short-stretch cohesive inelastic bandage
The short-stretch bandage has a high dynamic pressure and a low resting pressure because of the small amount of elasticity in the bandage. When mobility occurs, in a short-stretch bandage, the pressure will increase as the muscles contract, but the bandage does not expand. This is beneficial as it promotes venous return by assisting the calf muscle pump. When the person wearing a short-stretch bandage elevates the limb (resting), sub-bandage pressure is reduced because the lack of elasticity in the bandage prevents recoil.
|
Product |
Pack size |
Pecos item code |
Single item price (Pecos) |
Single item price (Drug Tariff) |
|
Short Stretch Cohesive Inelastic Bandage – LATEX – Actico |
||||
|
Bandage Compression Short Stretch Cohesive 6cm x 6m Latex |
1 |
093335 |
BEST PRICE
|
|
|
Bandage Compression Short Stretch Cohesive 10cm x 6m Latex |
1 |
038114
|
BEST PRICE
|
|
|
Bandage Compression Short Stretch Cohesive 12cm x 6m Latex |
1 |
217953 |
BEST PRICE
|
|
|
Short Stretch Cohesive Inelastic Bandage - Latex Free – Comprilan |
||||
|
Bandage Compression Short Stretch 8cm x 5m |
1 |
218134 |
BEST PRICE
|
|
|
Bandage Compression Short Stretch 10cm x 5m |
1 |
228591 |
BEST PRICE
|
|
|
Bandage Compression Short Stretch 12cm x 5m |
1 |
218158 |
BEST PRICE
|
|
2 Layer bandage system
Treatment is aimed at correcting, as much as possible, the long-term complications of chronic venous insufficiency. Compression therapy systems applied externally to the lower leg increase pressure on the skin and underlying structures to counteract the force of gravity. This can help to relieve the symptoms in the lower limb by acting on the venous and lymphatic systems to improve removal of fluid (blood and lymph) from the limb. Their use calls for an expert knowledge of the elastic properties of the products and experience in the technique of providing careful graduated compression. Incorrect application can lead to uneven and inadequate pressures or to hazardous levels of pressure. In particular, injudicious use of compression in limbs with arterial disease has been reported to cause severe skin and tissue necrosis (in some instances calling for amputation).
|
Product |
Pack size |
Pecos item code |
Single item price (Pecos) |
Single item price (Drug Tariff) |
|
2 Layer Bandage System – UrgoKTwo Full Compression - Latex Free |
||||
|
18-25 cm ankle (10 cm) |
1 |
230648 |
BEST PRICE |
|
|
25-32 cm ankle (10 cm) |
1 |
230655 |
BEST PRICE |
|
Medicated bandages
These may require a 48-hour patch test using a small amount of the bandage applied to the skin as paste bandages can be associated with hypersensitivity reactions and should be used with caution.
Zinc paste bandage can be used with compression bandaging, short stretch bandaging and type1 and 2 bandages for the treatment of leg ulceration. Useful for weeping exudate and/or blistering to the skin by protecting and preventing further damage from maceration. Can be applied to skin around wounds and left insitu whilst allowing regular dressing changes to continue.
Zinc paste bandages are also used with coal tar or ichthammol in chronic lichenified skin conditions such as chronic eczema (ichthammol often being preferred since its action is considered to be milder). Can also be used where hypersensitivity reactions have occurred with Viscopaste as the milder Ichthammol may be more easily tolerated.
Follow the manufacturer’s instructions when applying medicated bandages to prevent constriction of the blood flow. If a preservative-free brand is required, Zipzoc® is available.
|
Product |
Pack size |
Pecos item code |
Single item price (Pecos) |
Single item price (Drug Tariff) |
|
Zinc Paste Bandage BP (Viscopaste®)7.5 X 6M |
1 |
Only available from pharmacy or by prescription |
n/a |
BEST PRICE
|
|
Zinc Paste and Ichthammol Bandage BP (Ichthopaste®) 7.5cm x 6m |
1 |
Only available from pharmacy or by prescription |
n/a |
BEST PRICE
|
Tubular bandages
These are available in different forms according to the function required of them.
Elasticated tubular bandages are particularly suitable for retaining dressings, maintaining bandages on difficult parts of the body or for soft tissue injury. They are not suitable as the only method of applying pressure to an oedematous limb or to a varicose ulcer since they exert non-graduated and often inadequate pressure. Inappropriate use of elasticated tubular bandages can cause pressure damage and affect the shape of the limb, causing indents to fragile tissue.
Tubular stockinette
These garments can be prescribed to assist the wet-wrapping of children and some are suitable for protecting areas to which creams and ointments have been applied. They should not be used when potent corticosteroids are applied. They can be used under orthopaedic casts, compression bandaging, short stretch bandaging and type 1 or type 2 bandages to protect the skin from the bandage layers.
A 100% cotton stockinette should be considered for patients with sensitivities to synthetic materials.
|
Product |
Pack size |
Pecos item code |
Single item price (Pecos) |
Single item price (Drug Tariff) |
|
Lightweight Elasticated Surgical Tubular Stockinette Bandage – Clinifast – used for dressing retention and comfortable support |
||||
|
Bandage Adult Trunk Beige 10m |
1 |
211418
|
BEST PRICE
|
Tariff size: 17.5cm x 1m |
|
Bandage XLarge Limb Yellow 10m |
1 |
211449 |
BEST PRICE
|
Tariff size: Trunk Child Yellow Line 5m |
|
Bandage Large Limb Blue 10m |
1 |
211456
|
BEST PRICE
|
Tariff sizes: 7.5cm x 1m 7.5cm x 3m 7.5cm x 5m |
|
Bandage Medium Limb Green 10m |
1 |
211425
|
BEST PRICE
|
Tariff sizes: 5cm x 1m 5cm x 3m 5cm x 5m |
|
Bandage Elasticated Tubular Stockinette – Blue Dot – used for dressing retention without constriction or compression (Not on Drug Tariff – alternative CLINIgrip) |
||||
|
Bandage Tubular Stockinette BP 4.5cmx10m Size A |
1 |
214082
|
BEST PRICE
|
Tariff sizes: 4.5cm x 0.5m - £0.61 4.5cm x 1m - £1.11 |
|
Bandage Tubular Stockinette BP 6.25cmx10m Size B |
1 |
214099
|
BEST PRICE
|
Tariff sizes: 6.25cm x 0.5m- £0.61 6.25cm x 1m - £1.10 |
|
Bandage Tubular Stockinette BP 6.75cmx10m Size C |
1 |
214105
|
BEST PRICE
|
Tariff sizes: 6.75cm x 0.5m - £0.65 6.75cm x 1m - £1.17 |
|
Bandage Tubular Stockinette BP 7.5cmx10m Size D |
1 |
214112
|
BEST PRICE
|
Tariff sizes: 7.5cm x 0.5m - £0.66 7.5cm x 1m - £1.19 |
|
Bandage Tubular Stockinette BP 8.75cmx10m Size E |
|
214129
|
BEST PRICE
|
Tariff sizes: 8.75cm x 0.5m £0.74 8.75cm x 1m - £1.26 |
|
Bandage Tubular Stockinette BP 10cmx10m Size F |
1 |
214136
|
BEST PRICE
|
Tariff sizes: 10cm x 0.5m - £0.74 10cm x 1m - £1.26 |
|
Bandage Tubular Stockinette BP 12cmx10m Size G |
1 |
214143 |
BEST PRICE
|
Tariff sizes: 12cm x 0.5m - £0.77 12cm x 1m - £1.48 |
|
Net Stockinette for Retention – Surgifix – used for dressing retention in awkward places |
||||
|
Bandage Surgifix size 1 25M net stockinette |
1 |
099191
|
BEST PRICE
|
Not on Drug Tariff |
|
Bandage Surgifix size 2 25M net stockinette |
1 |
030576
|
BEST PRICE
|
Not on Drug Tariff |
|
Bandage Surgifix size 3 25M net stockinette |
1 |
061754
|
BEST PRICE
|
Not on Drug Tariff |
|
Bandage Surgifix size 4 25M net stockinette |
1 |
017164
|
BEST PRICE
|
Not on Drug Tariff |
|
Bandage Surgifix size 5 25M net stockinette |
1 |
091560
|
BEST PRICE
|
Not on Drug Tariff |
|
Bandage Surgifix size 6 25M net stockinette |
1 |
001811
|
BEST PRICE
|
Not on Drug Tariff |
|
Product |
Pack size |
Pecos item code |
Single item price (Pecos) |
Single item price (Drug Tariff) |
|
Surgical Adhesive Tape permeable plastic – CliniporeClear |
||||
|
Tape permeable plastic surgical 1.25cmx10m |
24 |
211685
|
BEST PRICE
|
Tariff size: 1.25cm x 5m - £0.35 |
|
Tape permeable plastic surgical 2.5cmx10m |
12 |
211692
|
BEST PRICE
|
Tariff size: 2.5cm x 5m - £0.59 |
|
Tape permeable plastic surgical 5cmx10m |
6 |
211708
|
BEST PRICE
|
Tariff size is: 5cm x 5m - £1.00 |
|
Surgical Adhesive Tape permeable non-woven– Finepore (Not on Drug Tariff – alternative Chemipore) |
||||
|
Tape permeable non-woven 1.25CM |
24 |
211647
|
BEST PRICE
|
|
|
Tape permeable non-woven 2.5CM |
12 |
211654
|
BEST PRICE
|
|
|
Tape permeable non-woven 5CM |
6 |
211661
|
BEST PRICE
|
|
|
Surgical Adhesive Tape non woven – Medipore (Not on Drug Tariff – alternative Primafix) |
||||
|
Tape surgical soft cloth 5cm |
1 |
213856 |
BEST PRICE
|
|
|
Tape surgical soft cloth 10cm |
1 |
213870
|
BEST PRICE
|
|
|
Tape surgical soft cloth 15cm |
1 |
213887
|
BEST PRICE
|
|
|
Product |
Pack size |
Pecos item code |
Single item price (Pecos) |
Single item price (Drug Tariff) |
|
Irrigation Solutions (Sterile Sodium Chloride 0.9%) |
||||
|
Normasol Sachet 25ml (pk 25) |
25 |
036424 |
BEST PRICE
|
25x25ml |
|
Dressing Pack - Rocialle |
||||
|
Universal procedure pack with Medium glove |
10 |
223718 |
BEST PRICE
|
Nurse it |
|
Non Woven Gauze Swabs 4 ply N/S |
||||
|
Swab gauze N/W fabric N/S 10x10cm 4ply |
200 |
211661
|
BEST PRICE
|
Tariff pack of 100 |
Pain can affect everyone. The psychological impact of pain cannot be measured nor underestimated and is likely to affect healing. Poor techniques in wound care can further traumatise wounds leading to additional pain and slowed healing or other complications. However strategies can be adopted through which pain can be avoided or minimised.
Patients may experience pain as a result of:
- Products or techniques used to cleanse wounds
- Trauma to the tissues and surrounding skin when products are removed
- Skin excoriation from exudates or wound drainage
- Lack of empathy
- Failure to record patients earlier reports of pain
- Infection, which can exacerbate existing wound pain
- Poor technique when using compression bandaging
Careful wound assessment is required to expose the causes, type and severity of pain that the patient is experiencing. All practitioners have a professional responsibility to implement effective pain reducing strategies.
The correct dressing can aid comfort and reduce pain, especially during dressing change.
Emotional responses can also influence the perception of pain and the distress of having a wound. Clinically, pain, like wound types, can be classified as acute or chronic, but will be related to:
- The type of injury
- The location of the wound
- Patient perception and previous experience
- The healing process and approaches to wound management, e.g. choice of dressing and provision of analgesia.
Assessment of Pain
Accurate assessment depends on subjective reporting by the patient. Pain can be assessed effectively during ongoing therapy by asking the patient to rate his/her pain. It is recommended that a simple visual analogue scale is used. The patient should be asked if the pain is worse at any particular time or during a particular activity so that analgesic doses can be timed appropriately. Patients should be closely observed throughout the dressing procedure for reaction to treatment. (BPS)
Analgesia
Whatever analgesia is used in wound care, its effectiveness should be evaluated continuously. Failure to achieve pain relief may contribute to the depression and anxiety associated with chronic pain.
The type of analgesia to be used depends upon:
- The type of wound
- Whether the wound is acute or chronic
- The level of pain reported by the patient
- Patients individual circumstances e.g. other medicines, co-morbidities
Effective doses of analgesics should be given. In chronic pain, treatment should be given regularly to provide continuous pain relief. This is preferable to giving analgesics only whennecessary, allowing pain to recur before giving further treatment.
Analgesics should also be given in anticipation of pain, giving careful consideration to any activities which exacerbate pain. In the case of acute pain there is little time to titrate the dose against the patient’s response. Analgesics should be chosen according to assessment of the factors mentioned above.
The use of non-steroidal anti-inflammatory drugs (NSAIDs) eg aspirin, ibuprofen, Diclofenac etc, is common in treating minor injuries and in long-term inflammatory conditions. This is due to their action of inhibiting the production of prostaglandins (inflammatory mediators). If wound pain is ongoing it may not be appropriate to use an NSAID due to their side effects.
The WHO analgesic ladder forms the basis of many approaches to the use of analgesic medicines.
There are three essential steps on this ladder.
The WHO Analgesic Ladder 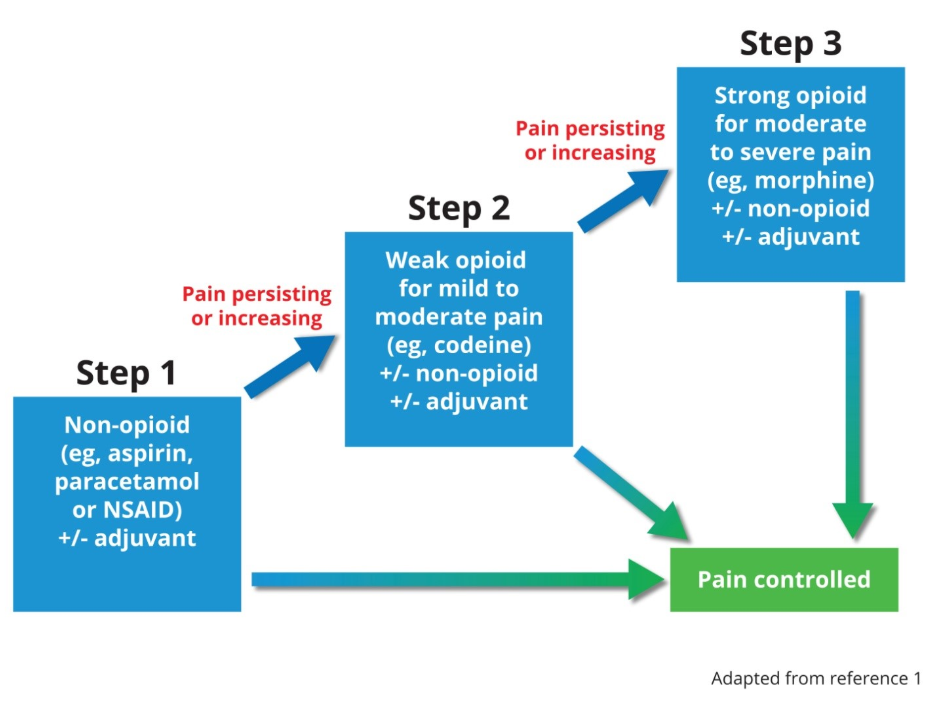
References
- Campbell, J. (1995) Making sense of pain management. Nursing Times 91, 34-35.
- An Independent Advisory Group (2004).
- Casey, G. (1998) The management of pain in wound care. Nursing Standard 13, 49-54.
- Dealey, C. (2005) The Care of Wounds, 3rd ed. Oxford: Blackwell Science.
- World Health Organization (1996) WHO Guidelines: Cancer Pain Relief, 2nd ed. Geneva, World Health Organization.
The principles of good skin care depend on:
- keeping the skin clean and dry
- avoiding the excessive use of soap as this can dry the skin
- using showers in preference to baths where possible
- keeping the skin moisturised
Assessment
The state of the skin surrounding a wound should be assessed at each dressing change. Observe for the following:
- dry skin - may break down and provide a portal for infection
- maceration - caused by poor management of exudates
- inflammation - consider contact sensitivity to dressings or infection
Emollients
Choice of emollient is very personal; it can mean the difference between treatment success and failure. Patients will not use a topical therapy that irritates their skin and they will be reluctant to use one that doesn’t feel right or suit their needs. Emollients are moisturisers that soothe and hydrate the skin. They are indicated for all dry or scaling disorders. Most are best applied after washing but their effects are short-lived so they must be applied frequently and regularly to maintain improvement. They should continue to be applied even after improvement occurs. Emollients should be applied in the direction of hair growth. Some ingredients may rarely cause sensitisation and this should be suspected if an eczematous reaction occurs.
There are different types of product available. These include ointments, creams, lotions and gels. Effectiveness depends upon the correct choice of product and correct use. Choice will depend upon:
- the severity of the condition
- patient preference
- the site of application
- cost of preparation
Ointments
Ointments are greasy and generally insoluble in water so can be difficult to wash off, and do not suit all patients. They are recommended as the first choice of formulation in most skin conditions and are particularly useful for chronic dry conditions. Examples: Epaderm ointment, liquid and white soft paraffin ointment 50:50.
Creams
Creams are emulsions of oil and water and often contain an antimicrobial preservative . They are therefore more likely to cause both irritant and allergic reactions. For this reason creams are best avoided first line but are often more cosmetically acceptable for some patients. Creams can be better than ointments for some acute conditions due to a cooling effect as they evaporate from the skin. Example: Diprobase.
Gels
Gels also have a high-water content and produce a cooling effect on evaporation from the skin. They are suitable for use on the face and scalp. Example: Doublebase gel.
Barrier Preparations
Wounds which are heavily exuding or have friable surrounding skin are at risk of excoriation, epidermal stripping and maceration. A barrier can be used on the surrounding skin prophylactically to protect the skin. Barrier preparations should be reapplied at dressing changes.
NB - Sudocrem should not be used as a barrier in wound management.
Some patients will experience leakage of urine or accidents, so the skin can become exposed to urine and faeces. This can cause the skin to become wet and soggy (moisture lesion) and can develop into a painful rash. See HIS: pressure ulcer grading and excoriation tool. Incontinence dermatitis can cause distress and embarrassment. The use of oil-based barrier creams interferes with the pad absorbency and can lead to urine staying in contact with the skin. The use of water-based creams is advised.
Signs and symptoms
Will appear as a red and painful skin rash, usually irregular in shape and may/may not be over bony prominences. It may also be itchy and flaking, crusty or weeping.
Treatment
- Genital Skin Care Leaflet
- Regular inspection of the skin is vital by a suitably trained practitioner.
- Use the lowest possible absorbancy of pad.
- Moisture causes damage to the skin so keeping the skin dry is imperative. Pat the skin dry and avoid rubbing.
- Appropriate product selection and correct fitting is vital - training is available to ensure knowledge and skills are up to date.
- If skin is wet, it can be cleaned with plain water – soap can make the skin dry and irritated.
- Any faeces on the skin must be removed promptly .
- Foam cleansers can be useful if used correctly
- spray foam directly onto skin.
- leave for 1-2 minutes to loosen and breakdown debris on the skin.
- remove with dry wipe only.
- always adhere to manufacturer’s instructions for each product as this may vary.
- Barrier creams can help protect the skin – it is important to use a water-based cream so as not to interfere with product absorbency. Please see wound management guidelines and formulary product list for the barrier creams. The use of Sudocrem or other oil-based creams affects the absorbent ability of the pad.
- A film type of gel/dressing would not be used routinely in a skin care regime but may be used when extra protection of the skin is required. It can be used on "at risk" skin as a prophylactic and also on broken skin to form a barrier and a protection for adjacent skin. If skin is damaged, then a review of the skin care regime is required.
- Talcum powder should not be used in conjunction with body worn continence products. When dampened by sweat or urine, talc can form encrustations, which may cause discomfort and increase potential for frictional damage.
- An all-in-one product which is too large or too small may cause leakage and skin irritation. With a shaped product, fixation pants or patients own pants must be close-fitting.
Contact
If the problems persist and further guidance is required, please contact Jenny Munro AP Physiotherapist & Independent Prescriber - Continence nhsh.continencecare@nhs.scot.
As a general rule, routine cleansing of wounds to remove bacteria or to reduce infection is unlikely to be effective (Miller and Gilchrist 1997).
Wound cleansing may be advocated to remove contaminants in the following instances:
- To remove visible debris after a wound has occurred to aid assessment.
- To remove excess slough and exudates.
- To remove any remaining dressing material.
- Prior to obtaining a microbiology swab.
- Adult Wood Cleansing Guideline NATVN.
Frequent washing of wounds is unnecessary and undesirable.
Cleansing Solutions
In the past wounds were cleansed with antibacterial solutions. Studies comparing the effectiveness of antibacterial solutions to tap water, normal sodium chloride 0.9% and distilled water have foundno difference in lowering bacterial countand no increased incidents of infection(Dire & Welsh 1990; Rodeheaver et al. 1982). Antiseptic solutions have been reported to cause tissue damage and hinder the healing process and are unlikely to be effective (Hellewell et al. 1997).
Sterile sodium chloride 0.9%, which is an isotonic solution, does not impede the healing process, cause allergic reactions or alter the bacterial flora of the skin. It should be used in the following situations, where tap water is not recommended:
- On exposed bone or tendon.
- On skin or bypass graft.
- For severely immunosuppressed patients.
Methods of Cleansing
Irrigation is the cleansing mechanism recommended for removal of contaminants. Scrubbing causes pain and local tissue oedema, which decreases host defences. Vigorous cleansing may however be necessary, in some instances, to remove grease and dirt from traumatic wounds which, if left in situ, can cause unsightly tattooing of the skin (Miller & Glover 1999).
Summary
- Does the wound need to be cleansed? If not, don’t do it.
- Always warm the irrigation fluid being used. Cooling the wound inhibits cell mitosis.
- Never use cotton wool or gauze swabs to clean wounds as they damage granulating tissue and shed fibres, which increase the risk of infection.
References
- Dire, D.J. & Welsh, A.P. (1990) A comparison of wound irrigating solutions used in the emergency department. Annals of EmergencyMedicine 19, 704-708.
- Hellewell, T.B., Major, D.A., Foresman, P.A., Rodeheaver, G.T. (1997) A cytotoxicity evaluation of antimicrobial and non-antimicrobial woundcleansers. Wounds 9, 15-20.
- Miller, M. & Gilchrist, B. (1997) Understanding Wound Cleaning and Infection. London: Macmillan.
- Miller, M. & Glover, D. (1999) Wound Management: theory and practice. London: Emap Healthcare Ltd.
- Rodeheaver, G., Bellamy, W., Kody, M. et al. (1982) Bactericidal activity and toxicity of iodine-containing solutions in wounds. Archives ofSurgery 117, 181-186.
|
Tissue Type |
Indicator /descriptor |
Management aims |
Treatment options |
|
|
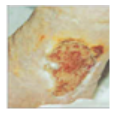
Necrotic
|
Necrotic layer of devitalised tissue. |
|
Leg For autolytic debridement use: Hydrogel if exude is low. Fibrous hydrocolloid if exude is high. Primary Wound contact layer as per formulary list above previous dressing options |
|
|
Heel
|
||||
|
Sloughy
|
Layer of dead tissue. |
|
Alginate Gel
|
Fibrous hydrocolloid
|
|
Granulating
|
Red, velvety and moist. |
|
Primary wound contact layer
|
Polyurethane foam film dressings (no border)
|
|
Epithelialising
|
Red/pink, new skin cover extending from wound edges, may have islands of new epithelium in main areas of the wound bed. |
|
Primary wound contact layer
|
Polyurethane foam film dressings (no border)
|
Other considerations (key points)
- Simple primary wound contact layersare recommended in the management of uncomplicated venous ulcers.
- Bacterial swabs should only be taken where there is clinical evidence of infection.
- Antimicrobial dressings should be considered if indicated and advised by trained practitioners.
- Sharp debridement should only be carried out by appropriately trained practitioners.
References
Leg ulceration is common and prevalence increases with age. Approximately 1% of the population will suffer from leg ulceration at some point in their lives. Venous ulceration is the most common type of leg ulceration followed by arterio-venous ulceration and arterial ulceration. Accurate, holistic assessment of the ulcer including ankle brachial pressure index (ABPI) or assessment by a trained practitioner is the key to successful treatment and must be performed prior to the application of any compression therapy.
Patients with the following features should be referred to the appropriate specialist at an early stage of management: see Referral Pathways. We also have a new nugget on the intranet to support appropriate referral.
- Suspicion of malignancy
- Peripheral arterial disease (ABPI<0.8)
- Rheumatoid/vasculitis
- Suspected contact dermatitis or dermatitis resistant to topical steroids
- Diabetes mellitus
- Atypical distribution of ulcers
- Non-healingulcerafter 4 to 6 months
- May benefit from surgical intervention such as foam sclerotherapy
|
Type |
Indicator/descriptor |
Management aims |
Treatment options |
|
Venous
|
|
|
|
|
Arterio-Venous
|
|
|
Following specialist referral and under close supervision options may include:
Compression hosiery Class 1 with specialist advice |
|
Arterial
|
|
|
Specialist Vascular referral for all patients. Compression bandaging or Short-stretch cohesive inelastic bandagemust NEVER be used on arterial leg ulcers. The use of the Primary Wound Contact Layer is the best dressing to use on arterial ulcers butit is important not to miss the opportunity to improve the blood supply if possible. This will be more effective than any change of dressing. Consider orthotic footwear in ulceration to the foot, heal or ankle areas to avoid further damage from excess pressure. |
Other considerations
- Latex free brands of compression bandages should be used routinely.
- Compression and short stretch cohesive inelastic bandaging should only be applied by staff with appropriate training and in accordance with the manufacturer’s instructions.
- Patients should be assessed for skin complications within 24 to 48 hours following initiation of compression.
- Recurrence of venous ulcers can be substantially reduced by continuous application of compression hosiery.
- Below knee graduated compression hosiery is recommended to prevent recurrence of venous leg ulcers. Patients should be offered the highest class of compression which they can tolerate to prevent recurrence, with 2 pairs being supplied on a 6 monthly basis.
- Nutrition: There is evidence to demonstrate that adequate levels of protein, fat, carbohydrates, vitamins and trace elements are necessary in wound healing, especially in collagen formation and maturation.
- Pain control is an important factor in patient concordance to any treatment regime and a programme of pain management should be tailored to the needs of each individual patient.
- Associated dermatitis which has not responded to topical steroid therapy should be considered for a referral to Dermatology.
Further Reading
- European Society for Vascular Surgery – 2017 ESVS Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases.
- Revision of the CEAP Classification for Chronic Venous Disorders: consensus statement. Journal of Vascular Surgery; 40: 1248-1252.
- Understanding Compression Therapy: EWMA.
- www.vascularsociety.org.uk.
References
The following dressings can be used on the direction of a specialist in secondary care eg Dermatologist, Plastic Surgeon, Vascular Surgeon, Leg Ulcer Specialist Nurse, Vascular Specialist Nurse and Plastic Surgery Nurse Specialist. A non-catalogue Pecos order would be required with a clear indication of who has agreed to order a non-formulary compliant product.
Debrisoft: For use in long standing hyperkeratotic tissue and diabetic foot ulceration where standard formularyproducts have failed.
Prontosan irrigation fluid 1litre with adapter: for use with Veraflo irrigation system. Available on product formulary and Pecos.
Cutimed Sorbact: This dressing binds and inactivates pathogenic bacteria and fungi which are removed from the wound at every dressing change thus preventing reproduction of micro-organisms. Useful in managing biofilms, the wound bioburden and vascular wounds. Suitable for fungal infections in the groin, skin folds or between digits particularly in diabetic patients.
Jelonet: For use in infective and irritant dermatitis, to soothe and calm the affected area whilst allowing free drainage of exudate into a secondary dressing. Also indicated for use where irritant contact dermatitis caused by reactions to wound care products and/or wound exudates produces high exudate levels This dressing contains Paraffin and is flammable. Patients should be made aware and advised to wash clothes if seepage occurs and caution near open flames.
Larvae: Larvae therapy is appropriate in the management of wounds requiring rapid debridement of slough and necrotic tissue including leg ulcers, pressure ulcers, infected surgical wounds, diabetic foot ulcers and burns. Please contact pharmacy for availability of prescription.
Medicated Bandages - Zinc Paste Bandage BP (Viscopaste®) 7.5 x 6M, Zinc Paste and Ichthammol Bandage BP (Ichthopaste®) 7.5cm x 6m:may require a 48-hour patch test using a small amount of the bandage applied to the skin as paste bandages can be associated with hypersensitivity reactions and should be used with caution.
Zinc Paste Bandage can be used with compression bandaging, short stretch bandaging and type1 and 2 bandages for the treatment of leg ulceration. Useful for weeping exudate and/or blistering to the skin by protecting and preventing further damage from maceration. Can be applied to skin around wounds and left insitu whilst allowing regular dressing changes to continue.
Zinc paste bandages are also used with coal tar or ichthammol in chronic lichenified skin conditions such as chronic eczema (ichthammol often being preferred since its action is milder). Can also be used where hypersensitivity reactions have occurred with Viscopaste as the milder Ichthammol may be more easily tolerated.
Follow the manufacturer’s instructions when applying medicated bandages to prevent constriction of the blood flow. If a preservative-free brand is required, Zipzoc® is available.
Lymphoedema is swelling primarily due to lymphatic insufficiency. This may be an inherited or congenital primary lymphoedema, or an acquired lymphoedema that is secondary to cancer treatment, or non-cancer causes including surgery and infection.
Some individuals develop chronic swelling (chronic oedema) that starts with a non-lymphatic cause. Several factors may contribute to a sustained increase in interstitial fluid, overwhelming and compromising the lymphatic system, often leading to lymphoedema, and delayed wound healing.
For further management advice and referral pathway, please refer to lymphoedema guidelines and Compression Hosiery Formulary.
|
Condition |
Typical appearance |
Description |
Management |
|
Lymphorrhoea |
|
Leakage of lymph from the skin surface. |
Daily washing with non-soap cleanser and careful drying. Daily application with emollient, as Highland Wound Formulary, to prevent maceration of surrounding skin. Dress with primary wound contact dressing and high absorbency wound pad, one layer of blue/yellow line lightweight elasticated surgical tubular stockinette bandage (Clinifast), wool bandages and further layer of compression (refer to Wet Legs care plan). Reference:
|
|
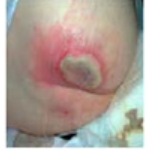
Cellulitis |
|
Infection of the deeper layers of skin and underlying tissue causing infected areas to look red and swollen and to feel painful and hot. |
Refer to cellulitis guideline. Avoid compression garments during the acute attack. However, they should be replaced as soon as the affected area is comfortable enough to tolerate them. Reference:
|
|
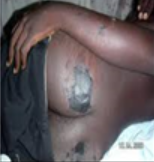
Hyperkeratosis |
|
Abnormal thickening of the outer layer of the skin. |
Daily washing with soap substitute and careful drying to reduce the risk of fungal infections. Use pre-moistened debridement wipes (UCS wipes) on hyperkeratotic areas to gently remove skin plaques. Daily application with emollient, as Highland Wound Formulary. Simple emollients need to be applied 2-3 times a day so complex emollients may be more effective when using garments or bandaging. Reference:
|
|
Papillomatosis |
|
The development of warty growths on the skin consisting of dilated lymphatics and fibrous tissue. |
Daily washing with non-soap cleanser and careful drying to reduce risk of fungal infections. Use pre-moistened debridement wipes (UCS wipes) on hyperkeratotic areas if necessary. Daily application with emollient, as Highland Wound Formulary. Simple emollients need to be applied 2-3 times a day so complex emollients may be more effective when using garments or bandaging. Compression with either custom-fit flat knit garment or inelastic adjustable compression wrap depending on patient dexterity. |
|
Lymphangiectasia |
|
Dilation of lymph vessels; may appear as blister-like protuberances on the skin. |
Daily washing with non-soap cleanser and careful drying. Daily application with emollient, as Highland Wound Formulary. Simple emollients need to be applied 2-3 times a day so complex emollients may be more effective when using garments or bandaging. Compression with either custom-fit flat knit garment or inelastic adjustable compression wrap depending on patient dexterity. |
|
Skin folds |
|
Skin folds around the ankle, mid calf and below knee frequently develop, sometimes as a result of poor bandaging or incorrect garments. |
Daily washing with non-soap cleanser and careful drying. Particular attention is required to reduce the risk of fungal infection and/or cellulitis. Daily application with emollient, as Highland Wound Formulary. Simple emollients need to be applied 2-3 times a day so complex emollients may be more effective when using garments or bandaging. The limb requires re-shaping by the application of under-padding with wool bandage, padding out deficits in the limb to equalise the sub-bandage pressure, followed by one or more layers of short stretch bandage applied in a spiral. Two-layer systems may also be effective. Inelastic adjustable compression wraps may be used in place of bandages. |
|
Lipodermatosclerosis |
|
Thickening and hardening of the subcutaneous tissues of the lower leg with brown discolouration of the skin; associated with chronic venous insufficiency; in severe cases lymphatics become damaged. |
Daily washing with non-soap cleanser and careful drying. Daily application with emollient, as Highland Wound Formulary. Simple emollients need to be applied 2-3 times a day so complex emollients may be more effective when using garments or bandaging. Compression with either custom-fit flat knit garment or inelastic adjustable compression wrap depending on patient dexterity. Further reading: |
Further reading
- Partsch, H. & Mortimer, P., Compression in leg wounds, British Journal of Dermatology, 173, p359-369.
For the Wet Legs Care Plan click here
|
Instructions for Toe Bandaging Adapted from Lymphoedema Network Wales Chronic Oedema ‘Wet Legs’ Pathway |
|||
|
Problem: Oedema affecting the dorsum of the foot and/or toes |
|||
|
Goal: To manage toe oedema OR to prevent toe oedema |
|||
|
Instructions: Record: CHI, Primary Nurse, Date |
|||
|
Wash leg in emollient and apply moisturising cream. |
Standard toe bandaging in situ
Demonstration of folding the toe bandage
Microfine toe cap (toes cut to correct length) |
||
|
Apply 4 or 5cm bandage and anchor with a loose turn around the base of the forefoot. |
|||
|
Take the bandage across the dorsum of the foot up to the big toe, wrap around the base of the toenail then anchor with a turn around the base of the forefoot. Use light tension only. |
|||
|
When applying the bandage to each toe - ensure all skin is covered and up to the base of the toenail as shown. The bandage may need to be folded in half for shorter toes. You do not need to bandage the little toe. |
|||
|
Toe caps are an alternative to toe bandaging; Microfine toe caps are available from Haddenham Healthcare or custom fit from Medi and Jobst. |
|||
|
Apply multi layer lymphoedema bandaging if required or follow care plan for leg ulcer management as directed. |
|||
Pressure ulcers are damage caused by extrinsic factors (pressure, shearing forces, friction) and intrinsic forces (illness, age, nutritional status, drug therapy). The toes, heels, sacrum and ischial tuberosites are at most risk of developing pressure ulcers. All pressure damage, regardless of grade, is reportable via Datix incident reporting. Moisture lesions should not be classified as pressure ulcers. For further guidance please refer to HIS: Pressure ulcer excoriation tool and for further product information see Incontinence Dermatitis.
|
Type |
Indicator/ Descriptor |
Management Aims |
Treatment Options |
|
|
1st Line |
2nd Line |
|||
|
|
Early warning sign - Blanching erythema Areas of discoloured tissue that blanch when fingertip pressure is applied, and the colour recovers when pressure released, indicating damage is starting to occur but can be reversed. On darkly pigmented skin blanching does not occur and changes to colour, temperature and texture of skin are the main indicators. |
To prevent further skin damage from pressure, shear friction or moisture particularly over bony prominences. |
If not already in place, commence patient centred care plan to reduce risk of pressure damage |
|
|
|
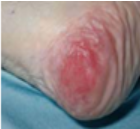
Grade 1 |
To prevent further skin damage from pressure, shear, friction or moisture particularly over bony prominences. |
If not already in place, commence care plan to reduce risk of pressure damage. |
|
|
|
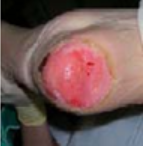
Grade 2 |
To protect. To promote new tissue growth. Not to be confused with skin tears (see burns, incontinence dermatitis, moisture and excoriation as these havedifferent treatment aims. |
Hydrocolloid. |
Specialist silicone wound dressing. |
|
|
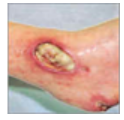
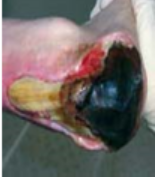
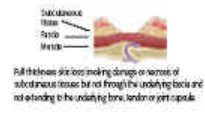
Grade 3 Subcutaneous fat may be visible but not bone, muscle or tendon. Slough may be present but does not obscure the depth of tissue loss. May include tunnelling and undermining. |
Offloading area to pressure is essential. The tissue may recover or deterioration may be rapid. Pressure damage should be closely observed at each positional change to note any change in appearance. To remove any dead tissue. To promote new tissue growth. Care plan prepared and SSKIN Bundle in place Refer to Tissue Viability using referral form and clinical photography consent form at nhsh.tissueviability@nhs.scot. |
Treatment to be prescribed after referral to TVNs. |
|
|
|
Grade 4 |
Offloading area to pressure is essential. The tissue may recover, or deterioration may be rapid. Pressure damage should be closely observed at each positional change to note any change in appearance. To remove any dead tissue. Should ideally have sharp debridement (this should only be performed by those who are trained to debride wounds). To promote new tissue growth. May require plastic, vascular, orthopaedic or general surgery teams. Care plan prepared and SSKIN Bundle in place. Refer to Tissue Viability using referral form and clinical photography consent form at nhsh.tissueviability@nhs.scot. |
Treatment to be prescribed after referral to TVNs. |
|
|
|
Ungradable Full thickness skin/tissue loss where the depth of the ulcer is completely obscured by slough and/or necrotic tissue. Until enough slough and necrotic tissue is removed to expose the base of the wound the true depth cannot be determined. It may be a Grade 3 or 4 once debrided. Once grade can be established this must be documented. |
To rehydrate eschar and remove any dead tissue. Caution: If circulation is compromised on the heel/foot consider specialist referral before attempting debridement eg vascular or plastics. Offloading area to pressure is essential. The tissue may recover, or deterioration may be Rapid. Pressure damage should be closely observed at each positional change to note any change in appearance. Care plan prepared and SSKIN Bundle in place. Refer to Tissue Viability using referral form and clinical photography consent form at nhsh.tissueviability@nhs.scot. |
||
|
|
Suspected Deep Tissue Injury Epidermis will be intact, but the affected area can appear purple or maroon or be a blood filled blister over a dark wound bed. Over time this skin will degrade and develop into deeper tissue loss. Once grade can be established this must be documented. |
Offloading area to pressure is essential. The tissue may recover, or deterioration may be rapid. Pressure damage should be closely observed at each positional change to note any change in appearance. Care plan prepared and SSKIN Bundle in place. Refer to Tissue Viability using referral form and clinical photography consent form at nhsh.tissueviability@nhs.scot. |
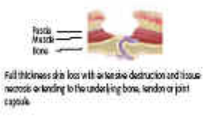
*For all Pressure Ulcers: In the event of tissue necrosis extending to underlying bone, tendon or joint capsule, advancing cellulitis or ulcer related sepsis, surgical intervention may be necessary. See Tissue Viability Referral Pathway. |
|
|
|
Combination Lesions These are lesions where a combination of pressure and moisture contribute to the tissue breakdown. They still need to be graded as pressure damage as above but awareness of other causes and treatments is needed. See Excoriation & Moisture Related Skin Damage Tool and Incontinence Dermatitis section. |
Offloading area to pressure is essential. The tissue may recover or deterioration may be rapid. Pressure damage should be closely observed at each positional change to note any change in appearance. Refer to Incontinence Dermatitis section. Referral to Advanced Nurse, Continence:nhsh.continencecare@nhs.scot. |
|
|
Other considerations
- It may be necessary to exclude potential osteomyelitis when dealing with deep wounds.
- For wound infection see relevant section.
- For cavities wounds and sinus tracts see relevant section.
In the event of tissue necrosis extending to underlying bone tendon or joint capsule, advancing cellulitis, or ulcer related sepsis, surgical intervention may be necessary, refer to vascular or plastic surgery.
End of life: Management aims should be focused on patient comfort and quality of life.
Provide the patient with the Pressure Ulcer patient information leaflet available on the Intranet.
References
- NHSH Patient Information Leaflet: Preventing Pressure Ulcers.
- European Pressure Ulcer Advisory Panel and National Pressure Ulcer Advisory Panel. Treatment of pressure ulcers: Quick Reference Guide. Washington DC: National Pressure Ulcer Advisory Panel; 2009. Available at: www.epuap.org.
- National Institute for Health and Clinical Excellence (NICE). Pressure Ulcers; September 2005.
- NHS Highland Pressure Ulcer Prevention and Management Policy.
- Standards for the Prevention and Management of Pressure Ulcers.
Integrated Pathway for Diabetes & ALL Foot Ulceration
At first presentation ensure all referrals are directed to podiatry services with a completed referral form.
The goal of any foot ulcer management is to:
- Create ulcer free days
- Give ulcer remission
- Limb salvage
- Quality of life
- Decrease mortality
Adherence to locally established guidance and protocols may reduce length of hospital stay and major complication rates. Wound healing and foot saving amputations can be achieved in the presence of multidisciplinary foot care teams. International Diabetes Federation recommends that every individual with diabetes receive the best possible care (IWGDF, 2019).
Risk Factors
The following factors can cause vulnerability to foot ulcers:
- previous history of foot ulceration
- neurological factors
- ischaemia
- foot deformity/posture
- callus
- oedema/lymphoedema
- neglect
Categorisethe diabetic foot using risk stratification related to SCI-Diabetes foot risk stratification tool (SIGN 116, 2010, updated May 2021).
Diabetes foot ulcer classification, utilising TEXAS classification system aids recording of wound characteristics, improves communication, predicts clinical outcomes and supports audit & research. The Scottish Diabetes Foot Action Group advocates the use of this tool when classifying foot ulcers.
The Scottish adaptation of the European Pressure Ulcer Advisory Panel (EPUAP) Pressure Ulcer Prevention Tool is used in NHS Highland when classifying pressure wounds.
Assessment
- Vascular – consider clinical features; perform appropriate vascular tests in line with local vascular pathway.
- Infection – clinical features; differential diagnosis; sampling; AMWD.
- Pressure/load distribution– appropriate footwear; appropriate device for load distribution.
- Evidence based wound care assessment & management– debridement; dressings.
- Holistic management– optimise management of relevant co-morbidities. Support peoples’ health & wellbeing through making every contact count.
Differential Diagnosis
|
Neuropathic Ulceration |
Ischaemic Ulceration |
|
|
If pain is experienced in a neuropathic foot, consider the possibility of infection or Charcot foot. A significant number of diabetes foot ulcers will be of mixed aetiology which can be identified with appropriate assessment.
Standards of Care



Treatment Aim
- to prevent deterioration of the wound
- to promote rapid closure with the minimum of tissue damage
- to prevent recurrence of foot ulceration
- to liaise with MDT foot/vascular if required.
References
- https://iwgdfguidelines.org/guidelines/guidelines/
- Management of Infection Guidance NHS Highland (2016)
- https://www.sign.ac.uk/assets/sign116.pdf
- https://www.woundsinternational.com/resources/details/best-practice-guidelines-wound-management-diabetic-foot-ulcers
- https://tam.nhsh.scot/healthcare-professional-information/therapeutic-guidelines/diabetes/diabetic-complications/diabetic-foot-infections/
- https://tam.nhsh.scot/healthcare-professional-information/antimicrobial-guidance/skin-soft-tissue/diabetic-foot-infection/
Referral
Referrals from any health care professional are accepted. For governance purposes they must include a podiatry referral (self-referral, sci- referral or hospital referral) to the local podiatry generic email address and include written communication eg letter, completed SCI-Diabetes comments/ulcer management section, e-clinic proforma.
Digital images should also be included with the referral.

NHS HIGHLAND HOT FOOT
High-risk foot Service covering all NHS Highland foradvice email: nhsh.diabetesfootclinic@nhs.scot
Podiatry Contacts
Argyll & Bute:
- Campbeltown -Tel: 01586 788977, email:nhsh.podiatrycampbeltown@nhs.scot
- Helensburgh - Tel: 01436 655132
- Lochgilphead -Tel: 01546 462199, email:nhsh.podiatrymidargyll@nhs.scot
- Oban - Tel: 01631 788977, email: nhsh.podiatryoban@nhs.scot
- Rothesay & Dunoon - Tel: 01700 501529, email:nhsh.cowal.podiatrychronicwounds@nhs.scot
North:
- Caithness East - Tel: 01955 880435, email: nhsh.podiatrynorthdivision@nhs.scot
- Caithness West & North West Sutherland - Tel: 07748 761899, email: nhsh.podiatrynorthdivision@nhs.scot
- East Sutherland - Tel: 01408 633157, email: nhsh.podiatrynorthdivision@nhs.scot
South and Mid (including Raigmore Hospital):
- South and Mid - Tel: 01463 723250, email:nhsh.southandmidpodiatry@nhs.scot
West:
- Lochaber - Tel: 01397 709800, email:nhsh.podiatrylochaber@nhs.scot
- Skye & Lochalsh & Wester Ross - Tel: 01478 613200, email:nhsh.podiatryslwr@nhs.scot
AVULSION/ABLATION OFTOENAILS
Background
The object of performing toenail avulsion/ablation is to: alleviate discomfort; reduce risk of sub-ungual ulceration; prevent recurrence and so treat the problem. Avulsion may be carried out on part of the nail or the whole nail. A combination of avulsion with or without matrix destruction may be performed. Podiatry destroys the matrix using chemical ablation, and this should prevent regrowth of the nail. Studies show chemical avulsion dramatically reduces the rate of symptomatic recurrence. Podiatry uses a 10% sodium hydroxide solution to carry out this chemical ablation.
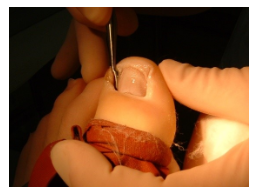
Use of sodium hydroxide (NaOH)
- 10% NaOH solution is applied to the area being treated
- Chemical ablation procedures have a slightly higher chance of becoming infected
- Chemical ablation procedures may produce delayed healing.
- Drainage is common for 3 to 4 weeks post-operatively
- Any query should be directed to the clinician who has performed the procedure.
Hypergranulation
Hypergranulation is a relatively common complication of ingrown toenails. Hypergranualtion may distort the end of the toe, however a small amount may be left after nail avulsion without concern and will regress following removal of the offending spicule of nail.
Post-Operative Management
Immediately Post-Operatively, with Significant Bleeding:
- Primary dressing: alginate dressing
- Secondary dressing: polyurethane foam film dressing
- Retaining dressing: surgical stockinette
Immediately Post-Operatively, with no Significant Bleeding:
- Primary dressing: polyurethane foam film dressing
- Retaining dressing: surgical stockinette
initial post-operative dressing change by patient, following chemical ablation, should be performed at 24 to 48 hours. If the dressing is adhered to the wound, it may be soaked off with tap water or sterile saline depending on availability.
- packing of the wound should be discouraged as this prevents drainage of exudate.
- packing causes the dressing to become hard and can cause discomfort when removal is attempted.
- further redressing should be governed by wound status eg slough, granulation etc.
References
- Eekhof J, Wyk, BV, Neven AK, van der Wouden, JC (2012) Interventions for Ingrowing toenails
- FernandezR., Griffiths,R (2012) Water for wound cleansing
- http://emedicine.medscape.com/article/1126725-treatment
- http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD001541.pub3/full
Burns management (NHS Highland intranet access required)
Infection is the invasion of living tissue by micro-organisms. The number of micro-organisms and their degree of pathogenicity determine the establishment of infection. The presence of bacteria does not always indicate that a wound is infected. All chronic wounds are colonised with bacteria, but they only become problematic when healing is compromised. Wound stasis or deterioration may be caused when the host defenses are unable to resist the bacterial load. Internal and external factors related to the patient’s condition must be taken into consideration when planning care of an infected wound. Factors such as immunosuppression, poor nutritional status, metabolic disease, ischemia or presence of a foreign body will affect a host’s ability to control bacterial load.
|
Type |
Management aims |
Treatment options |
|
|
1st Line |
2nd Line |
||
|
Colonised |
|
See WMF section according to wound type. |
See WMF section according to wound type. |
|
Locally Infected |
|
Honey Iodine Flaminal
|
Silver |
|
Spreading Infection |
|
Honey Iodine |
Silver |
|
Systemic Infection |
|
Honey Iodine |
Silver |
Other considerations
Diagnosing Clinical Infection - Resources to guide the management of suspected infection in chronic wounds (healthcareimprovementscotland.org.
Signs of infection include erythema, pain, swelling and localised heat. In addition, wound infection can be identified with the following potential signs: increased discharge, delayed healing, wound breakdown, pocketing at the base of the wound, epithelial bridging, unexpected pain or tenderness, friable granulation tissue, discolouration of the wound bed, abscess formation or malodour.
Inflammatory response may be inhibited in some people. Those who are immunosuppressed or have diabetes mellitus may not display typical signs and symptoms of wound infection. It may be necessary to exclude potential osteomyelitis when dealing with deep wounds.
Wound Swabs
Wound swabs should only be taken for infection that is spreading or where systemic infection is suspected.
- Prior to obtaining a wound swab, wounds should be cleansed as normal.
- Take swab from deeptissue (as close to the wound bed as possible).
- The swab should be moved across the wound surface in a zigzag motion, at the same time as being rotated between the fingers.
- Where possible, submit actual tissue samples - these may be sent in universal containers.
- A specimen of pus is more valuable than a pus swab when sampling abscesses at incision and drainage.
- When delay in transit is unavoidable, keep at room temperature or refrigerate at 4°C.
Antimicrobial Dressings
Use of antimicrobial dressings should be limited to two to three weeks unless there is clear clinical justification to continue for longer. If the wound is unchanged at three weeks, it is recommended that an alternative topical antimicrobial agent is used. If the wound begins to show further signs of infection, the use of a systemic antibiotic should be considered.
Honey Dressings
Honey dressings have antimicrobial and anti-inflammatory properties and promote autolytic debridement. This osmotic effect may cause pain and analgesia may be required. Patients with diabetes should have their control monitored whilst using topical honey, especially when applied to large areas. Honey dressings should be avoided in patients with extreme sensitivities to honey, bee stings or bee products.
Iodine Dressings
Iodine dressings release free iodine when exposed to wound exudate which acts as an antiseptic on the wound surface. System absorption may occur from large wounds or with prolonged use. Caution in those with severe renal impairment or history of thyroid disease. Contra-indicated in children, those receiving lithium, thyroid disorders, pregnancy and breastfeeding. Inadine® has the propensity to dry out and adhere to the wound surface and indicates frequent dressing changes.
Other Antimicrobial Dressings
Flaminal® is a dressing with enzymatic antimicrobial activity. Experience with these dressings is limited and therefore it may be prudent to limit their use to mild infection until their place in therapy has been established.
Silver Dressings
Silver ions exert an antimicrobial effect in the presence of wound exudate and have broad antimicrobial activity. Practitioners should ensure good wound bed contact and conformability for full benefit from these dressings.
Topical antibiotic creams are not recommended.
Systemic antibiotics
Asprescribed by medical team.
Malodour
Maladourcan be the most distressing symptom for patients, family and care givers. See Fungating Wounds for management advice.
Secondary Dressings
See WMF section according to wound type for appropriate choice of secondary dressings.
References
- Wounds UK. Best Practice Statement. The use of topical antiseptic/antimicrobial agents in wound management. Aberdeen May 2011.
- World Union of Wound Healing Societies (WUWHS). Wound Infection in Clinical Practice. An International Consensus. London: Medical Education Partnership Ltd. International Wound Journal: Vol 5; 3, 2008.
- National Prescribing Centre. Evidence-based prescribing of advanced wound dressings for chronic wounds in primary care. MeReC Bulletin 2010; 21(01): 1-7.
- Silver dressings – do they work? Drug & Therapeutics Bulletin. 2010; 48(4): 38-42.
- Wounds International. Ten top tips for taking a wound swab. Available at Wounds International.
As with all complex wounds a full assessment to determine the cause and extent of the wound is essential. “A wound sinus is a discharging blind-ended tract that extends from the surface of an organ to an underlying area or abscess cavity”. The cause of a sinus must always be determined by in-depth assessment. A cavity wound may be chronic or acute and falls into the categories described below.
|
Type |
Indicator/descriptor |
Management aims |
Treatment options |
|
Sinus
|
Discharging, blind-ended track that extends from the surface of the skin to an underlying abscess/cavity. May be caused by infection, liquefaction or a foreign body. |
|
Hydrogel Fill track if exudate is low (using a syringe) Fibrous hydrocolloid or alginate If exudate is moderate to high. |
|
Secondary dressing foam or silicone. |
|||
|
Cavity
|
A cavity wound may be acute or chronic. Surgical cavities are generally clean cavities with a healthy bed. Cavities can be present in a range of aetiologies (pilonidal sinus, pressure ulcers and leg ulcers are examples). |
|
Hydrogel Where there is tracking or undermining. |
|
Grade 3
|
Secondary Dressing foam or silicone. |
||
|
Grade 4
|
Other considerations
The amount of packing inserted into the wound should be documented to ensure it is all removed at the next dressing change.
Rope should be inserted very loosely as a wick to facilitate drainage and not cause a back flow of exudate into the body cavity. A 2cm tail should be left out with the wound to enable easy removal. Wounds should not be overfilled.
Sinus: Often end in an abscess/cavity which contains foreign material. This needs to be removed and healing promoted or the sinus is likely to become chronic.
Patient Assessment
- Establish the site and extent of tissue damage
- Consider barrier film to prevent maceration from exudate
- Nutrition
- Pain management
- Surgical intervention
- Multi-disciplinary approach
If wound infection is suspected refer to the Infection section.
References
- Butcher M (1999), Management of Wound Sinuses, Journal of Wound Care, Oct, 8: 9-1999; 415-453.
- NHS QIS. Prevention and Management of Pressure Ulcers. Best Practice Statement, March 2009.
Overgranulation can sometimes occur in the latter stages of healing and clinical action can help reduce the granulation.
|
Type |
Indicator/descriptor |
Management aims |
Treatment options |
 |
Overgranulation, also referred to as proud flesh, hypergranulation, hypertrophic granulation, hyperplasia of granulation tissue or wound oedema, occurs at the proliferative stage of the wound healing process. It presents clinically as granulation tissue raised above the level of the surrounding skin. The presence of overgranulation tissue prevents progress to the maturation stage of the wound healing process and so delays wound healing. Early recognition and treatment are essential to wound progression and healing. |
|
Examine the wound bed carefully; it is sometimes possible to see and remove dressing fibres and other potential irritants. Identify wound infection, a key cause of inflammation in wounds. Overgranulation can be a symptom of wound colonisation.
|
Other considerations
- Avoid occlusive dressings.
- If overgranulation fails to respond to any of the above topical steroids may be of use - refer to Advance Practitioner Dermatology or Advanced Practitioner Tissue Viability.
- Eliminate malignancy.
- Overgranulation is normally transient and will often resolve itself as the wound contracts (Dunford, 1999). Adopting a “wait and see” policy may therefore carry fewer implications for the patient than some of the other options discussed.
- Surgical excision: re-traumatises the wound, re-initiating the wound healing cycle. Undertaken in theatre as last resort.
References
- Cooper, R., 2007. Steroid therapy in wound healing. In Free Paper. EWMA Conference. Glasgow.
- Dunford, C (1999) Hypergranulation Tissue. Journal of Wound Care 8 (10) 506-7.
- Stephen-Haynes, J. and Hampton, S., 2010. Achieving effective outcomes in patients with overgranulation. Wound Care Alliance UK, pp.1-10.
- Hampton,S (2007) Understanding over granulation in tissue viability practice. British Journal of Community Nursing,September 2007, vol./is.1219 (S24-30), 1462 – 4753.
Fungating wounds are caused either by a local tumour infiltrating the skin, or by metastatic spread from the primary tumour. Malignant fungating lesions are an immense challenge. Management goals vary depending on the stage of the underlying cancer, the patient’s prognosis, and the individual’s own goals and wishes. In some cases, the aim is to arrest tumour growth, but in many situations fungating tumours occur at the end of life, and treatment is completed in a palliative setting that focuses on comfort and maintenance of the best possible quality of life for that patient and their family. In either case it is important to remember that the symptoms produced by a fungating wound are often as distressing as the wound itself. Management focuses on alleviation of distressing symptoms including pain, cutaneous irritation, exudate, bleeding and odour.
|
|
Indicator/descriptor |
Management aims |
Treatment options |
|
|
Exudate Wound exudate is produced as a normal part of the wound healing process. In fungating wounds the exudate produced can be excessive. This is thought to be due to increased permeability of blood vessels within the tumour and secretion of vascular permeability factor by tumour cells. |
|
Heavy exudate |
|
|
Bleeding Blood vessels can be disrupted by the infiltration of tumour cells which can lead to bleeding at the wound site. Traumatic dressing changes can also lead to bleeding. |
|
An alginate may be applied to wounds with a small amount of bleeding but should be used with caution in fragile tumours as they may cause bleeding. A silicone wound contact layer should be used as the primary dressing to prevent adherence to the wound. |
|
|
Malodour Malodour is associated with necrotic tissue that supports the growth of anaerobic bacteria, and the presence of volatile fatty acids in the wound. Stagnant exudate, infection and fistula formation are also contributing factors. Malodour is reported to be the most distressing symptom from the patients perspective. |
|
Metronidazole gel - apply to clean wound 1-2 times daily and cover with non-adherent dressing. Charcoal dressing should be used for light to moderate exuding wounds. |
|
|
Skin irritation |
|
Barrier cream may provide relief. Zinc oxide paste prescribed by pharmacy can be used to prevent peri-wound maceration. A hydrogel dressing can also reduce itching and promote comfort. |
|
|
Pain Assessment provides a means to determine the underlying cause of the pain, eg infection, pressure on structures, heightened anxiety, invasion of nerves. There are several types of pain associated with malignant wounds: deep pain, neuropathic pain and superficial pain related to procedures. Dressing removal has been found to be the time of greatest pain for those with chronic wounds. |
|
A short-acting analgesic such as Oramorph® may be given 20 to 30 minutes prior to dressing changes. Where nerve pain exists, adjuvant therapies may be of benefit. The use of morphine or diamorphine mixed with a hydrogel (to form a 0.08– to 0.1% mixture) and applied topically to the wound has been found to be an effective method of pain relief in ulcerating wounds, including fungating wounds. |
Other considerations
- Exudate - In selected wounds with high exudate either stoma appliances or absorbent pads over a non-adherent contact layer may be the most appropriate dressing method.
- Bleeding - Bleeding may be controlled by careful removal of dressings moistened first with warmed normal saline.
- Skin irritation - Advice to the patient regarding the use of light, cool clothing and bathing may be of some benefit. Irritation caused by radiotherapy may respond to topical hydrocortisone – seek specialist advice.
- Pain - Chemotherapy, radiotherapy, hormone therapy or a combination of these anti-cancer therapies may help to shrink the wound by destroying malignant cells, reducing pressure on nerves and other structures and decreasing the area of exposed tissue. This may have a significant effect on the level of pain experienced by the patient – seek specialist advice regarding appropriate analgesia.
- Dressing choice - Dressing choice for fungating wounds should not only be based on the wound characteristics but also should have minimum bulk, conform to the body to prevent leakage, conformable and cosmetically acceptable to the patient.
References:
- Bergstrom, KJ. (2011). Assessment and Management of Fungating Wounds, J Wound Ostomy Continence Nurse, 38(1); 31-37
- Grocott, P et al (2012). Malignant Wound Management in Advanced Illness: New Insights. Current Opinion in Supportive and Palliative Care. Review, 7(1); 101-105.
- Da Costa Santos, CM et al (2010). A Systematic Review of Topical Treatments to Control the Odour of Malignant Fungating Wounds, Journal of Pain and Symptom Management, 39(6); 1065-1076.
- Graves, LM. (2013). Providing Quality Wound Care at the end of Life. Journal of Hospice and Palliative Nursing, 15(2); 66-74.
- Alexander, S. (2009). Malignant Fungating Wounds: Epidemiology, Aetiology, Presentation and Assessment. Journal of Wound Care,18(7); 273-280.
- Seaman, S. (2006). Management of Malignant Fungating Wounds in Advanced Cancer. Seminars in Oncology Nursing, 22(3); 185-193.
- Chrisman, CA. (2010). Care of Chronic Wounds in Palliative Care and End-of-Life Patients. International Wound Journal, 7(4); 214-235.
- Zeppetella G, Ribeiro MD. (2005). Morphine in intrasite gel applied topically to painful ulcer. J Pain Symptom Manag,29; 118-119.
- Healthcare Improvement Scotland Best Practice Statement: Skincare of Patients Receiving Radiotherapy (2010).
For Tissue Viability Referral form please click here (Copy and paste the link into a new browser window. NHS Highland intranet access required).
For Product Complaint form please click here (Copy and paste the link into a new browser window. NHS Highland intranet access required).
1. Why have a formulary for these products?
The WMG&F provides guidance on the prescription and application of preferred tissue viability products for use in NHS Highland. In keeping with other NHS Boards, the NHS Highland Formulary is evidence based and takes into consideration patient acceptability as well as clinical and cost effectiveness. This aims to support the Highland Quality Approach to person centred care by minimising harm, variation and waste in the management of wound care.
The specialist products section should only be used where there is no suitable product for a particular wound type/clinical condition available from the standard primary and secondary product sections.
2. Where can the formulary and product list be found?
The WMG&F can be found on TAM under Formularies (and the Pink One), other formularies, Wound Management Guidelines and Formulary.
3. What benefits will adhering to these formularies bring?
Formularies are a standard tool for ensuring that product selection decisions are informed by best available evidence and are focussed on reducing clinical variation whilst ensuring best value is obtained from medicines and appliances, building clinical experience with a limited number of products. Adhering to formularies generates significant and recurrent savings that are needed to preserve other clinical services.
4. What if a product is not available on the formulary to meet the clinical needs of an individual patient?
A product from the formulary should meet clinical needs in the majority of cases; we are aiming for 90% of all wound products used to be formulary products.
Non formulary products should only be used when there is a good and justifiable clinical reason to do so. If, after a suitable trial of formulary products or the individual has a particular clinical need, there is no suitable formulary product then agreement should be sought from the Senior Charge Nurse/Team lead in conjunction with advice from an NHS Highland Tissue Viability Advanced Practice Nurse.
5. Should the updated product list be applied to the selection of products for people with new wounds or should it be applied to review products in use for people with existing wounds?
The new product list applies to the selection of products for all wounds and people who are being treated with non-formulary products should be switched to the formulary products as soon as is reasonably possible.
Where possible, make sure to use up any non-formulary products before switching to avoid waste.
6. What if a person is unhappy with a switch to a formulary product?
Listen to the person’s concern and try to understand their reasoning and provide an explanation of the rationale for the product change as part of NHS Highland policy. If the product meets the person’s need and they agree with the change, then proceed with use of the product. If however, the person remains concerned, share their concerns with the Senior Charge Nurse/Team Leader for resolution.
People who wish to make a complaint can do so via the NHS Highland Feedback Team.
7. Will the product list be reviewed?
Yes, the formulary products will be subject to regular review by the Tissue Viability Group. If you would like to suggest a product be added to/removed from the product list then refer to the Wound Formulary & Tissue Viability Section of the NHS Highland intranet.
8. District nursing teams are being encouraged to order wound management products they use from PECOS. Why is this?
There are a number of benefits to ordering products on PECOS:
- Pecos ordering provides nurses with a readily available supply of formulary products. Often there can be a delay in getting the right product to the individual when a prescription has to be written and dispensed.
- Community based nurses who are not prescribers themselves do not have to wait for a prescription to be issued by a GP or another prescriber.
- Generally, the same product is cheaper when supplied on PECOS compared to being supplied by issuing a primary care prescription.
- Products ordered via PECOS belong to the NHS whereas products prescribed for individuals become their property. So, a box of dressings can be split and used by a community nurse for different people rather than prescribing a separate whole box for each person. Prescribing whole boxes of products for individuals leads to considerable waste, especially if products are changed. Similar principles apply to all appliances.
9. How do I order products from PECOS?
The process is no different to ordering any other product via PECOS. PECOS catalogues have been updated to reflect formulary products. Local teams should order products in a way that suits their way of working eg setting up a system in your own team to monitor product use and stock levels and adjust quantities in future orders to reflect product usage and delivery schedules. Setting up a recurring requisition can speed up the ordering process.
10. Does this mean nurse or other prescribers can’t issue prescriptions for these products?
No, definitely not. There will be circumstances when there is a clinical need to use a non formulary product. If a product is required before the next delivery is due to your base, it may be necessary to have a prescription dispensed. Additionally, some products are not available via PECOS – these are highlighted in the product list.
11. Will all individuals have their wound management products supplied via PECOS?
Because of the advantages of ordering from PECOS for district nurse teams we recommend that all products being used by district nurses are ordered from PECOS. This includes people in residential homes that are having their wound managed by the district nursing team. People who are having their wound managed by someone other than a district nurse eg self care, by a nurse in a care home or by a practice nurse will still require to have their products supplied on prescription.
12. Will our team budget be affected?
Yes and No. Yes in that more cost will be recorded against your cost centre. And no because, at the end of an accounting period, costs will be reconciled and appropriately balanced with savings made on primary care prescribing.
13. I am a Senior Charge Nurse/Nurse Team Lead and am required to monitor my team’s compliance with the Formulary. How will I do this?
Monitoring of products ordered on PECOS can be reviewed from your team’s monthly finance reports. Reports detailing products that have been issued on prescription and formulary compliance are available at a district/locality level from your primary care lead pharmacist.
Click here to access form.