For use in Critical Care Areas for Adults only. Administer via central line only.
MECHANISM OF ACTION:
- Noradrenaline exhibits marked inotropic (beta-1) and vasopressor (alpha-1) effects.
- Half-life = 2.5 to 5 minutes.
USES:
- Hypotension in the absence of hypovolaemia (correct this first) eg in sepsis where there is low peripheral resistance.
- May be used as an alternative inotrope to adrenaline in cardiogenic shock eg in post-resuscitation phase.
CAUTIONS:
- Some brands contain sodium metabisulphite; caution if previous sensitivity.
- Caution in patients taking monoamine oxidase inhibitors, tricyclic antidepressants and linezolid due to risk of hypertension and arrhythmias.
PRESENTATION
- Ready-to-use vials containing 4mg in 50mL (single strength).
- Ready-to-use vials containing 8mg in 50mL (double strength).
- Ampoules containing 4mg in 4mL. Use only for preparing quad strength noradrenaline.
ADMINISTRATION:
- Use ready-to-use preparations wherever possible. Use caution when selecting strength.
- Always start treatment using single strength preparation and titrate to response. If the infusion is running at ≥ 10mL/hour, consider changing to a more concentrated preparation.
- If ready-to-use preparations unavailable, or preparing quad strength noradrenaline (16mg in 50mL, 320 micrograms/mL), dilute required volume of noradrenaline with glucose 5% to total volume and administer via syringe pump or volumetric infusion.
- Only use quad strength for patients requiring high infusion rates. Ready-to-use preparations are safer.
- For patients on high rates of quad strength noradrenaline, consider preparing in 100mL bag and giving via a volumetric pump.
- Inform pharmacy if using quad strength noradrenaline to ensure sufficient stocks available on unit.
- Must be given via a dedicated port.
|
Noradrenaline |
Single strength |
Double strength |
Quad strength |
|
|
Syringe pump |
Syringe pump |
Syringe pump |
Volumetric infusion |
|
|
Prescribe |
4mg in 50mL |
8mg in 50mL |
16mg in 50mL |
32mg in 100mL |
|
Ready-to-use preparation available |
Yes: use if available |
Yes: use if available |
No: prepare on ward |
|
|
Drug dose to be added |
4mg in 4mL |
8mg in 8mL |
16mg in 16mL |
32mg in 32mL |
|
Diluent to be added |
46mL glucose 5% |
42mL glucose 5% |
34mL glucose 5% |
68mL glucose 5% |
|
Final volume |
50mL |
50mL |
50mL |
100mL |
|
Final concentration |
80 micrograms/mL |
160 micrograms/mL |
320 micrograms/mL |
|
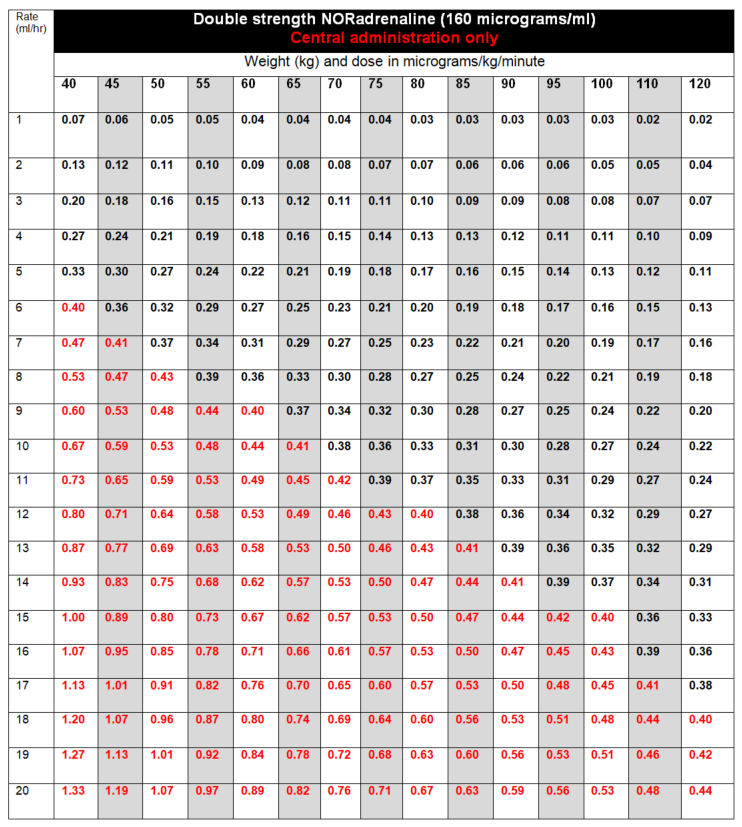
DOSE AND RATE:
- Titrate dose to achieve clinical target.
- The goal is to maintain an adequate blood pressure to ensure organ perfusion, and usually targeting a mean arterial pressure (MAP) of 65 to 75mmHg or target set by Consultant looking after patient. Dose required should be guided by heart rate (HR), blood pressure (BP), cardiac output, presence of ectopic beats and urine output.
- Infusions are usually started at 2 to 5mL/hour, and titrated by increasing or reducing the infusion rate by 1mL/hour to achieve target MAP.
- When stopping the infusion, reduce the rate of infusion gradually. Abrupt withdrawal can cause acute hypotension.
- If dose is escalating rapidly, or dose required to achieve clinical target exceeds 0.4 micrograms/kg/minute (highlighted in red in tables below) the doctor must be contacted to discuss further management. Options include:
- Addition of another therapy.
- Sedation level reviewed (ICU only).
- Cardiac output monitoring commenced (ICU only).
- Use of hydrocortisone IV 50mg four times daily (in patients with sepsis).
- A new upper limit may be set.
To calculate dose in micrograms/kg/minute, use tables below, NHS Injectable Medicines Guide (Medusa) for example calculations or the following calculation:
| Dose (micrograms/kg/minute) | = |
Rate (mL/hour) x Concentration (micrograms/mL) |
STABILITY:
- 24 hours.
- Do not allow the syringe or infusion to run out. A syringe or infusion can be made up to a maximum of one hour in advance and labelled clearly with contents and expiry. Refer to local nursing guidelines for switching over infusions or syringes.
EXTRAVASATION:
- The infusion has a low pH and extravasation is likely to cause venous irritation and tissue damage.
- Please refer to Peripheral extravasation injury (Non-cancer).
SIDE-EFFECTS
- Bradycardia (as a reflex to the increase in blood pressure).
- Arrhythmias.
- Ischaemia – peripheral, myocardial, renal or mesenteric.
MONITORING:
- Ensure ECG and blood pressure monitoring is in place. Invasive blood pressure monitoring is preferred as in hypoperfused or shock states with cool peripheries, non-invasive BP recordings are less reliable.
- Renal function and urine output.
- Monitor for tissue ischaemia or necrosis due to vasoconstriction.