For use in Critical Care Areas for Adults only. Administer preferably via central line (low pH).
MECHANISM OF ACTION:
- Positive inotrope and vasodilator, with little chronotropic activity.
- Selectively inhibits PEAK III cAMP phosphodiesterase isoenzyme in cardiac and vascular tissue, leading to an increase in intracellular ionised calcium and contractile force in cardiac muscle. This activity results in left ventricular afterload reduction, with an increase in cardiac output and reduction in total peripheral resistance.
- Half-life approximately 50 minutes in healthy volunteers; 1.7 to 2.7 hours in cardiac failure and up to 3.2 hours in renal impairment.
USES:
- Short-term treatment of severe congestive cardiac failure unresponsive to conventional maintenance therapy (not immediately after myocardial infarction).
- Acute heart failure including low output states.
- The use of milrinone in critical care is complicated by its long half-life (2 to 4 hours, prolonged in renal impairment), and adverse effects such as systemic hypotension.
CONTRA-INDICATIONS:
- Severe aortic or pulmonary valve disease, or hypertrophic subaortic stenosis. A drug with inotropic/vasodilator properties may aggravate outflow obstruction.
- Severe hypovolaemia.
CAUTIONS:
- The use of inotropic agents such as milrinone during the acute phase of a myocardial infarction may lead to an undesirable increase in myocardial oxygen consumption. Milrinone is not recommended immediately following MI.
- Supraventricular and ventricular arrhythmias have been observed. Ensure electrolyte derangements are corrected.
- Shortened AV node conduction can lead to increased ventricular rate in patients with uncontrolled atrial fibrillation or flutter. Consideration should be given to ceasing milrinone treatment if rate cannot be controlled with cardiac glycosides or other drugs.
- Improvement in cardiac output with resultant diuresis may necessitate a reduction in the dose of any diuretics co-prescribed.
- Potassium loss due to excessive diuresis may pre-dispose patients to arrhythmias. Potassium should be monitored closely and hypokalaemia corrected.
- Dose reduction required in renal impairment (see below).
PRESENTATION:
- Ampoules containing 10mg in 10mL.
ADMINISTRATION:
*Loading dose is not usually given due to risk of hypotension. Discuss with consultant*
Loading Dose
Administer loading dose by IV injection:
- Dilute the volume required for the loading dose (50micrograms/kg) to 10mL or 20mL of glucose 5% or sodium chloride 0.9% and give over 10 minutes.
Continuous infusion
- Prepare a 50mL solution containing milrinone 200 microgram/mL by diluting 10mL of milrinone 10mg/10mL with 40mL of glucose 5% (preferred) or sodium chloride 0.9%.
- Prescribe 10mg in 50mL, final concentration 200 micrograms/mL.
- Must be given via a dedicated port.
| Milrinone continuous infusion | 50mL syringe via syringe pump |
| Prescribe | 10mg in 50mL |
| Drug dose to be added | 10mg in 10mL |
| Diluent to be added | 40mL glucose 5% (preferred) or sodium chloride 0.9% |
| Final volume | 50mL |
| Final concentration | 200 micrograms/mL |
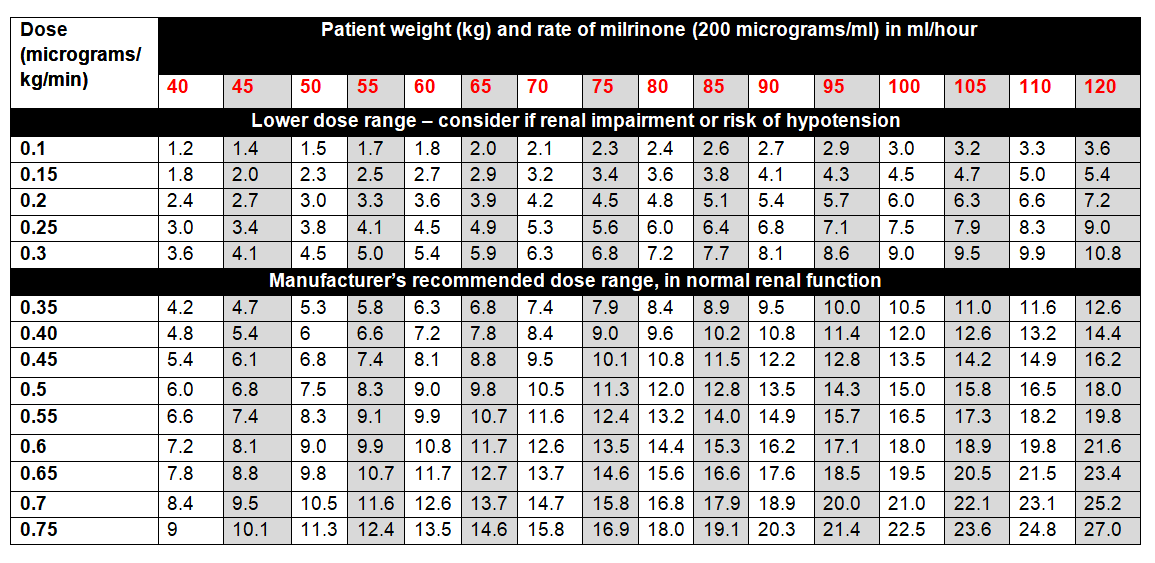
DOSE AND RATE:
Loading dose (if indicated, see above):
- 50 micrograms/kg.
Continuous infusion:
- Licensed dose: 0.375 to 0.75 micrograms/kg/minute in normal renal function. Adjust according to haemodynamic response. In practice, many units start at lower rates. See table below.
- Consider dosing according to adjusted body-weight in patients who are morbidly obese or over 120kg: mdcalc.com/ideal-body-weight-adjusted-body-weight.
- Dose needs to be adjusted for renal impairment due to risk of accumulation and hypotension. Note that eGFR is not applicable in AKI, use acute changes in renal function (eg urine output) to help guide dose adjustments. Start at lower end of dosing range and adjust according to haemodynamic response.
- Wean infusion slowly (over at least 2 to 4 hours), monitoring for clinical signs of inadequate cardiac output.
- Usual duration of therapy: 48 to 72 hours, maximum 5 days.
To calculate dose in micrograms/kg/minute, use table below or the following calculation:
| Dose (micrograms/kg/minute) | = |
Rate (mL/hour) x Concentration (micrograms/mL) |
STABILITY:
- 24 hours.
- Do not allow the syringe or infusion to run out. A syringe or infusion can be made up to a maximum of one hour in advance and labelled clearly with contents and expiry. Refer to local nursing guidance for switching over infusions or syringes.
EXTRAVASATION:
- Milrinone has a low pH and extravasation is likely to cause venous irritation and tissue damage. If given peripherally, use a large vein with monitoring for phlebitis. Resite catheter at first signs of inflammation.
- Please refer to Peripheral extravasation injury (Non-cancer).
SIDE EFFECTS:
- Supraventricular and ventricular arrhythmias have been observed. The infusion should be stopped if arrhythmias develop.
- Milrinone may induce hypotension as a result of vasodilatory activity. The rate of infusion should be reduced, or infusion stopped, if patients show excessive reduction in blood pressure.
- Mild thrombocytopenia.
MONITORING:
- ECG monitoring.
- Fluid balance.
- Renal function.
- Electrolytes (especially potassium).