For use in Critical Care Areas for Adults only. Administer via CENTRAL line.
For peripheral administration, see separate guideline.
MECHANISM OF ACTION:
- Marked inotropic (beta-1) effects which increase heart rate and force of contraction.
- Mild beta-2 and alpha-1 effects which decreases peripheral and pulmonary vascular resistance.
- It does not have direct effects on renal blood flow, but may increase renal blood flow due to an increase in cardiac output.
- Half-life = 2 to 3 minutes.
USES:
- Hypotension in the absence of hypovolaemia (correct this first).
- Cardiogenic shock.
- Other low cardiac output states eg post myocardial infarction.
CAUTIONS:
- Hypotension due to uncorrected hypovolaemia.
- Ischaemic heart disease (due to the impact on myocardial oxygen demand).
- Acute heart failure.
- Arrhythmias or rapid AF (may increase ventricular response rate).
- Marked obstruction of cardiac ejection eg idiopathic hypertrophic subaortic stenosis or hypertrophic obstructive cardiomyopathy.
- Elderly.
- Some brands contain sodium metabisulphite; caution if previous sensitivity.
- If administered continuously for more than 72 hours, tachyphylaxis may occur, requiring a dose increase.
- Contra-indicated in phaeochromocytoma.
PRESENTATION:
- 250mg in 50mL (5000 micrograms in 1mL) vials.
ADMINISTRATION:
- For central administration use the ready diluted preparation (250mg in 50mL) to provide a concentration of 5000 micrograms in 1mL.
- Administer via a syringe pump.
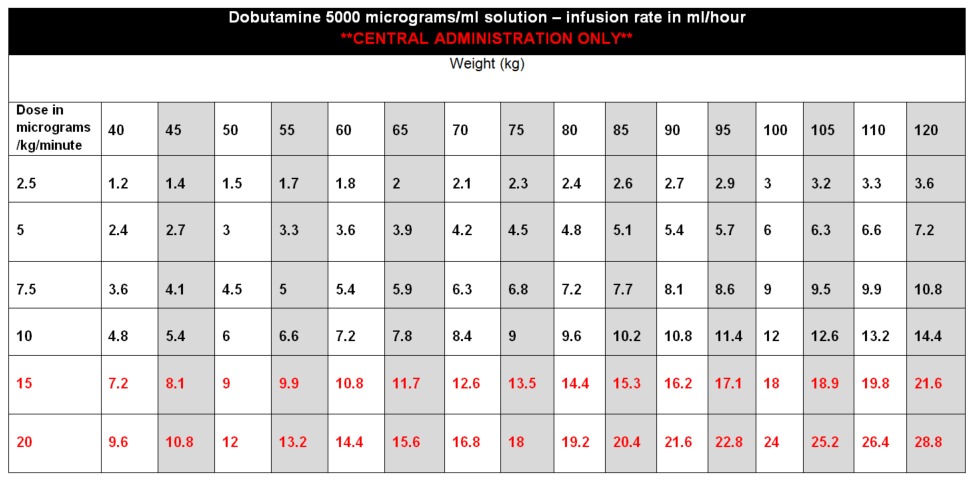
DOSE AND RATE:
- Dobutamine should be titrated to the cardiac indices set by the clinician. Dose required should be guided by HR, BP, cardiac output, presence of ectopic beats and urine output. In particular, patients receiving dobutamine should be monitored closely for tachyarrhythmias.
- The dose should be adjusted according to clinical response but would normally be kept in the range 2·5 to 10 micrograms/kg/minute, increasing to 20micrograms/kg/minute if needed.
- Doses above 10 micrograms/kg/minute should rarely be required.
- Adjust rate of dobutamine infusion to clinical effect with caution in obese patients.
- Do not allow infusion to run out. Prepare a new syringe or infusion before the previous one finishes. Refer to local nursing guidelines for switching over infusions or syringes. In clinically unstable patients, double-pumping may be required when switching over syringes; discuss with consultant.
- Must be given via a dedicated port.
- On discontinuation, reduce dose slowly rather than abruptly stopping.
To calculate dose in micrograms/kg/minute, use table below or the following calculation:
| Dose (micrograms/kg/min) | = |
Rate (mL/hour) x Concentration (micrograms/mL) |
STABILITY:
- 24 hours.
- Do not allow the syringe or infusion to run out. A syringe or infusion can be made up to a maximum of one hour in advance and labelled clearly with contents and expiry. Refer to local nursing guidelines for switching over infusions or syringes.
EXTRAVASATION:
- The infusion has a low pH and extravasation is likely to cause venous irritation and tissue damage.
- Please refer to Peripheral extravasation injury (Non-cancer).
SIDE EFFECTS:
- Hypotension.
- At higher doses, tachyarrhythmias.
- Alterations in serum potassium.
MONITORING:
- Ensure ECG and blood pressure monitoring is in place. Invasive blood pressure monitoring is preferred as in hypoperfused or shock states with cool peripheries, non-invasive BP recordings are less reliable.
- Daily electrolytes (especially potassium and magnesium) and fluid balance.
OTHER INFORMATION:
- Solutions may have a pink discolouration which may become darker with time, resulting from a slight oxidation of the drug. There is no significant loss of drug potency within the recommended storage time for solutions of the drug.