For use in Critical Care Areas for Adults only
Administer via CENTRAL line only
MECHANISM OF ACTION:
- Adrenaline exhibits primarily inotropic (beta-1) and vasodilator (beta-2) effects but some dose dependent vasopressor (alpha-1) effects are present at higher doses.
- Half-life = 1 to 3 minutes.
USES:
- Hypotension (second-line treatment for cardiogenic shock - after dobutamine).
- Bradycardia with adverse signs (shock, syncope, myocardial ischaemia, heart failure) and/or risk of asystole which has not responded to atropine (if external pacing unavailable or unsuccessful).
CAUTIONS:
- Hypotension due to uncorrected hypovolaemia.
- Tachycardia and arrhythmias.
- Narrow angle glaucoma.
- Phaeochromocytoma.
- Some brands contain sodium metabisulphite; caution if previous sensitivity.
PRESENTATION:
- Ampoules containing 5mg in 5ml (1 in 1000).
ADMINISTRATION:
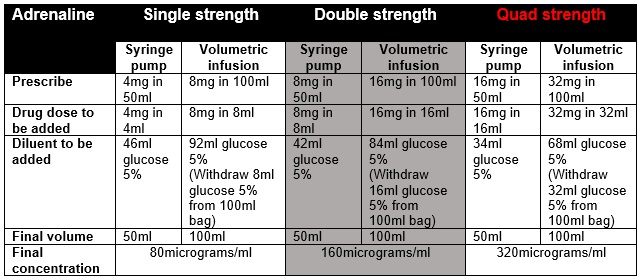
- Dilute required volume of adrenaline with glucose 5% to total volume and administer via syringe pump (when preparing in a bag to give via volumetric infusion, withdraw equivalent volume before adding adrenaline). The syringe must be clearly labelled.
- Always start treatment using single strength preparation and titrate to response. If the infusion is running at ≥10ml/hour, consider changing to a more concentrated preparation.
- Inform pharmacy if running quad strength adrenaline to ensure sufficient stock available on ward.
- Must be given via a dedicated port.
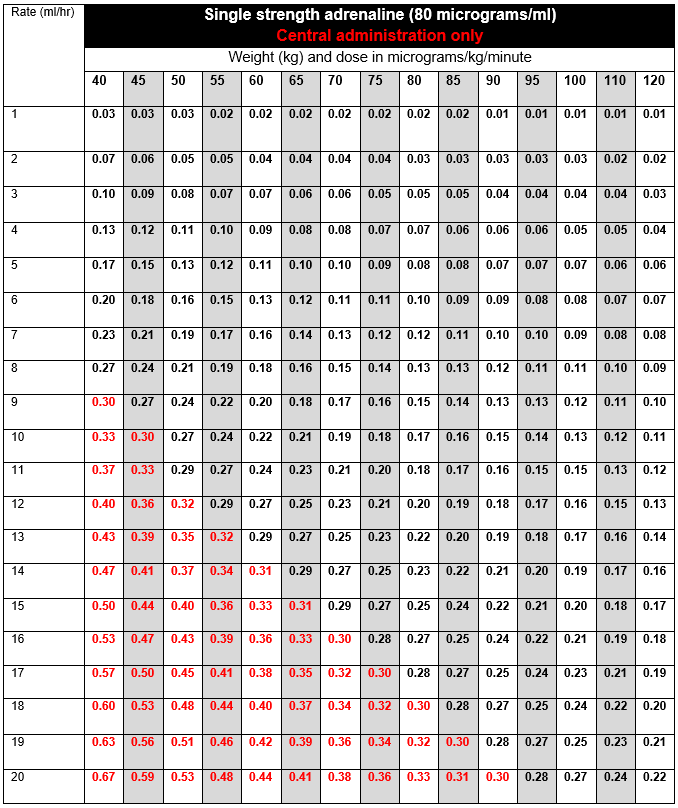
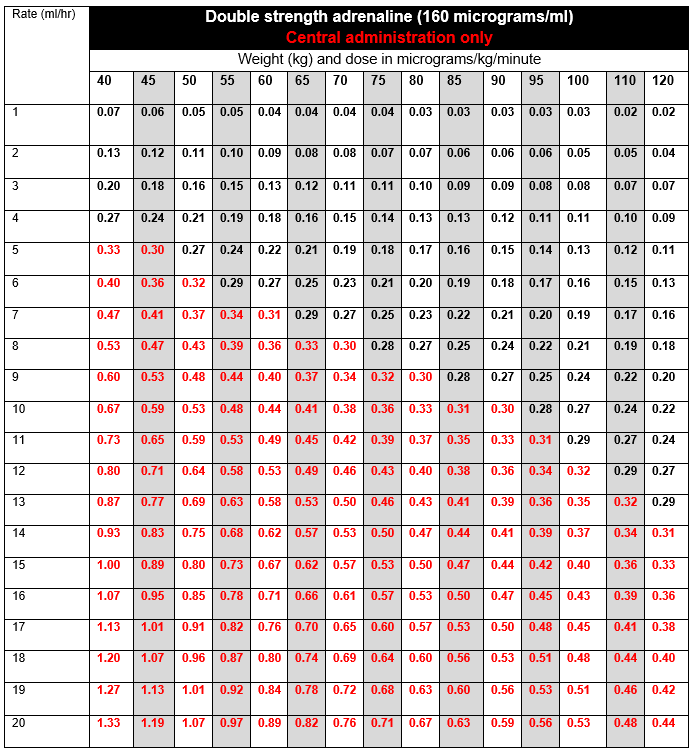
DOSE AND RATE:
- Titrate dose to achieve clinical target. The goal is to maintain an adequate blood pressure to ensure organ perfusion, and usually achieve a target mean arterial pressure (MAP) of 65 to 75 mmHg, as advised by the clinician. Dose required should be guided by heart rate (HR), blood pressure (BP), cardiac output, presence of ectopic beats and urine output.
- Infusions are usually started at 2 to 5ml/hour of single strength (80micrograms/ml), and titrated by increasing or reducing the infusion rate by 1ml/hour to achieve target MAP.
- If dose is escalating rapidly, or dose required to achieve clinical target exceeds 0.3 micrograms/kg/minute (highlighted in red in the tables below) the doctor must be contacted to discuss further management. Options include:
- Addition of another therapy.
- Sedation level reviewed (ICU only).
- Cardiac Output Monitoring commenced (ICU only).
- A new upper limit may be set.
To calculate dose in micrograms/kg/minute, use tables below or the following calculation:
| Dose (micrograms/kg/minute) | = |
Rate (mL/hour) x Concentration (micrograms/mL) |
STABILITY
- 24 hours.
- Do not allow the syringe or infusion to run out. A syringe or infusion can be made up to a maximum of one hour in advance and labelled clearly with contents and expiry. Refer to local nursing guidelines for switching over infusions or syringes.
EXTRAVASATION:
- The infusion has a low pH and extravasation is likely to cause venous irritation and tissue damage.
- Please refer to Peripheral extravasation injury (Non-cancer).
SIDE-EFFECTS:
- Angina, tachycardia, arrhythmias and palpitations.
- Hyperglycaemia.
- Increased lactate.
MONITORING:
- Ensure ECG and blood pressure monitoring is in place. Invasive blood pressure monitoring is preferred as in hypoperfused or shock states with cool peripheries, non-invasive BP recordings are less reliable.
- Pulse oximetry.
- Monitor for tissue ischaemia or necrosis due to vasoconstriction.
- Regular blood glucose monitoring.
- Lactate.