It is proposed to standardise the weaning of either Alfentanil or Remifentanil by:
1) Loading with Morphine,
2) Establishing a Morphine infusion and
3) Continuing the Remifentanil or Alfentanil infusion for at least 2 hours before stopping.

Cardiac patients in PICU: weaning Alfentanil and Remifentanil in ventilated post-operative cardiac patients in PICU.
This guideline has been written to standardise the smooth transition of alfentanil/remifentanil to morphine in PICU.
This aide-memoire is specifically for intubated, ventilated children in PICU during the first few hours post-cardiac surgery. It is intended for children with an Alfentanil or a Remifentanil infusion which has been started in theatre and which is still running when the child arrives in PICU. It is aimed at nursing and medical staff in PICU.
There is no published literature as such looking at transitioning between opiods in PICU. This suggested pathway is thus taken from current practice, expert opinion from within anaesthetics and intensive care and knowledge of the pharmacokinetics of the drugs involved. The pathway has been subjected to review within anaesthetics and PICU and comments and feedback have been incorporated.
Classically Morphine was the opiate of choice in cardiac anaesthesia. A total dose of 1mg/kg was used, divided into four equal doses, 0.25mg/kg in the anaesthetic room, 0.25mg/kg prior to sternotomy, 0.25mg/kg going onto bypass and 0.25mg/kg during rewarming. Morphine’s slow onset and long half-life (t½) has led to newer opiods being used intra-operatively.
Alfentanil and Remifentanil are potent opiod analgesics with rapid onset and clearance when compared to Morphine. They are used as infusions in theatre and in the immediate post-operative period. The analgesic actions of Alfentanil and Remifentanil modify the haemodynamic responses to surgical stress. Both are potent mu agonists with the characteristic side effects of all opiods, including profound respiratory depression. Their rapid offset can leave the patient in pain post-operatively.
Following cardiac surgery there will be an ongoing need for analgesia. Patients in PICU on conventional ventilation would normally be loaded with Morphine prior to the discontinuation of Alfentanil or Remifentanil.
Induction of Analgesia: 0.1 – 0.2 mg/kg. (Cardiac anaesthesia up to 1mg/kg in divided doses)
Maintenance of Analgesia by continuous infusion: 10 - 30ug/kg/hr
Continuation infusion in PICU: 10-40ug/kg/hr
Following IV bolus administration the onset time of Morphine is relatively slow taking 15-30 minutes. Morphine has a low lipid solubility (about 2.5% that of fentanyl) and at physiological pH is 80% ionized, which slows passage through the blood brain barrier. Its offset is also slower with a t½ of some 2 hours. One of its major metabolites morphine-6-glucoronide has virtually identical actions to morphine and an even longer t½.
Induction of Analgesia: 50-75 mcg/kg (Loading doses typically given in theatre over several minutes or as 10ug/kg increments)
Maintenance of Analgesia by Continuous Infusion: 0.5 to 3 mcg/kg/min
Continuation infusion in PICU0.5 – 4 ug/kg/min
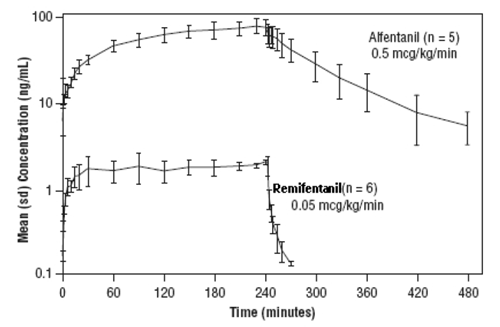
The pharmacokinetics of Alfentanil can be described as a three-compartment model with initial distribution t½ of 1 and 14 minutes; and a terminal elimination t½ of 90-111 minutes.
After a short intra-operative infusion the effective clinical half life of Alfentanil will be that of the phase 1 and 2 redistribution phases but after an infusion of a few hours it’s half life will be more dependant on the terminal elimination phase (fig.1).
Induction of Analgesia: 1 mcg/kg (Loading doses typically given in theatre over several minutes or as 0.1ug/kg increments)
Maintenance of Analgesia by Continuous Infusion: 0.1 to 4 mcg/kg/min (Average rate 1 to 1.5 mcg/kg/min)
Continuation infusion in PICU0.5- 1mcg/kg/min
The analgesic effects of Remifentanil are rapid in onset and offset. Remifentanil is an esterase metabolised opioid. A labile ester linkage renders this compound susceptible to hydrolysis by nonspecific esterases in blood and tissues. This gives Remifentanil an effective t½ of approximately 3 to 6 minutes. Due to the rapid offset of action of Remifentanil, no residual analgesic activity will be present within 5 to 10 minutes after discontinuation. This is irrespective of the duration of the infusion (fig.1). For this reason Remifentanil is rarely used for cardiac patients.
Fig 1.
For patients undergoing surgical procedures where postoperative pain is generally anticipated, alternative analgesics should be administered prior to discontinuation of Alfentanil or Remifentanil. The choice of analgesic should be appropriate for the patient's surgical procedure and the level of follow-up care.
It takes 3.3 half lives of a drug infusion to reach 90% of steady state concentration and 5 half lives to reach 97% of the final steady state concentration. In morphine’s case this would take some 6-10 hours. The use of a bolus dose allows the steady state to be reached more quickly as the infusion then merely has to maintain the drug concentration rather than building to a steady state (fig 2).
Fig 2
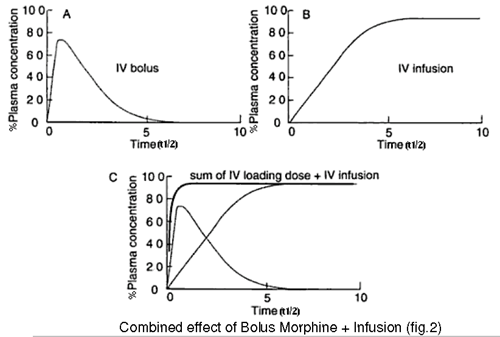
When using a combined Alfentanil/Morphine technique the anaesthetist will usually load the patient with a dose of Morphine in the period between rewarming and returning the patient to PICU, titrating the Morphine dose to the patient’s response.It is essential to ascertain from the anaesthetist whether a loading dose of morphine has been administered in theatre prior to the patient being transferred to PICU. This should be clearly documented on the handover form from theatre.
If the patient has received a loading dose of Morphine in theatre then they can be started on a morphine infusion on return to PICU as per normal indications at 10- 40ug/kg/hr of Morphine to keep the patient comfortable and to maintain light sedation (comfort score of 17-25).
If the patient has not received a loading dose of Morphine in theatre then a dose of 0.1mg/kg (0.05mg/kg in neonates) should be given at the start of the Morphine infusion. Further boluses of morphine can be administered as per standard practice from the syringe pump 1-2ml bolus (0.02 -0.04 mg/kg) if required.
If an Alfentanil or Remifentanil infusion is running from theatre the infusion should not be stopped immediately on arrival in PICU.
To allow a smooth transition from an Alfentanil/ Remifentanil infusion to a morphine infusion the Alfentanil/ Remifentanil infusion should be carried on at a rate of 0.5ug/kg/min ideally for at least 2hrs ( i.e. 1 morphine t½ ) to allow the morphine to reach a steady state. If there is less than 2 hours of Alfentanil/ Remifentanil in the pump then we can anticipate that additional morphine boluses will be required to allow an effective steady state of Morphine to be achieved more rapidly. A dose of 0.5ug/kg/min is suggested for both Alfentanil and Remifentanil for simplicity but it should be noted that this is a low dose of Alfentanil and a moderate dose of Remifentanil.