Near Infrared Spectroscopy (NIRS) Guideline (1072)

Near Infra-Red Spectroscopy (NIRS) is a non-invasive monitoring modality which measures regional tissue oxygenation and may provide an early warning of impaired tissue perfusion, prior to clinical presentation or elevation in biomarkers. This may afford the opportunity to enhance patient outcomes in a variety of different clinical situations; evidence for its use beyond cardiac surgery is continuously emerging (Tosh and Petteril, 2016).
The NIRS equipment provides information regarding regional tissue perfusion depending upon the site of the probe, whereas venous blood saturation (SvO2) informs us about total body perfusion.
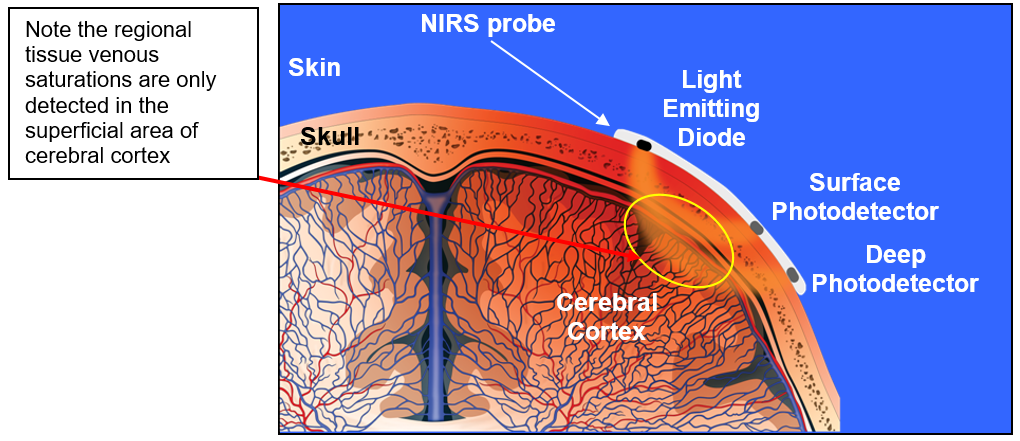
NIRS provides continuous, non-invasive monitoring of regional tissue oxygen saturation (%rSO2), therefore providing an indication of the balance between regional tissue oxygen delivery and extraction. The “NIRS value” is expressed as a % (rSO2) and reflects the amount of haemoglobin that remains saturated after passing through the local tissue bed.
Both animal and human studies have demonstrated that cerebral rSO2 correlates with continuous central venous oxygen saturation (ScVO2) (Gil-Anton et al, 2015, Marimon et al, 2012 and Rancucci et al, 2008), central venous saturation (SvO2) (Nagdyman et al, 2008, Bhutta et al, 2007 and Tortoriello et al, 2005) and jugular SvO2 (Ricci et al, 2006) and further studies have demonstrated rSO2 correlation with lactate (Kaufman et al, 2008 and Chakravarti et al, 2009).
The Equipment
1. INVOS NIRS monitor with 2 modules

2. Probes (single patient use)
- For Infants (<5kg): 3 probes of the same size
- For Children (>5kg-40kg): 2 probes of the same size and additional extensions cables– these are NOT disposable)

3. Hypafix Strips - NIRS probes should be attached using a piece of wide hypafix. The adhesive backing should not be removed, to facilitate easy skin inspection every 6 hours.
4. Grey cable and Phillips intellivue box, labelled ‘PICU NIRS’.
The Set-Up








Further Considerations:
Skin care
- Skin integrity should be routinely monitored by gently peeling back the hypafix to reveal the area under the probe every 6 hours.
- The probe must have good contact with the skin and secured with hypafix which can be renewed as required
- Avoid the use of any moisturising lotions on the skin and ensure the probe is closely adhered to skin.
Renewing Probes
- Probes are single patient use and should only be renewed if they stop working or has become visibly damaged.
Discontinuation of NIRS Monitoring
- Should only be done following discussion with the Consultant Intensivist and ought to be considered if:
- The patient is extubated successfully and demonstrates haemodynamic stability
- Care is re-orientated.
NIRS enables instant, non-invasive, continuous monitoring of regional tissue oxygenation at the bedside by measuring differential absorption of infrared light due to chromophores in biological tissue at multiple wavelengths (700-1000nm) (Marin and Moore, 2011). The INVOS system uses one emitter and two detectors, to measure changes in deoxygenated and oxygenated haemoglobin (HHb and HbO2) (Marin and Moore, 2011) in arterioles, venules and capillaries. The device is venous weighted (~85% venous, 15% arterial) and unlike pulse oximetry is not restricted by the absence of pulsatile flow and therefore can be used throughout cardiopulmonary bypass (Ghanayem et al, 2011).
A combination of two laws of physics, known as the Beer-Lambert-Law, regarding the absorption/penetration of light through different concentrations of a substance and different depths is utilised by the mathematical algorithms in the device software which process the readings to provide a regional oxygenation saturation value which is displayed both graphically and numerically.
Introduction
NIRS facilitates recognition of reduced or differential tissue perfusion which occurs during and following cardiac surgery during which time infants are at risk of Low Cardiac Output State (LCOS) which is associated with multi-organ dysfunction (i.e. acute kidney injury, cerebral injury), increased mortality and morbidity. NIRS may also have a role in the management of patients with sepsis, traumatic brain injury, post-cardiac arrest management and those on ECMO. Interpretation of NIRS allows optimisation of various management strategies to address the balance between tissue oxygen delivery (i.e. optimising oxygenation and haemoglobin concentrations, cautious use of fluid and inotropes) and reducing metabolic demand (i.e. pain relief, sedation and temperature control). In those with balanced cardiac conditions NIRS provides a valuable bedside indicator regarding Qp:Qs balance , which describes the magnitude of a cardiovascular shunt (i.e. normal = 1:1, left to right shunt = >1.0 and right to left shunt = <1.0).
Qp:Qs = (SatAorta – SatSVC*) / (SatPulmonaryVein – SatPulmonary Artery)
*In clinical practice venous sats from the SVC often utilised however, this should be SatMV i.e. true mixed venous sats obtained from the pulmonary artery.
INVOS NIRS Monitor
A number of NIRs devices are now available, although most clinical research studies have been conducted using the INVOS system (Schmidt et al, 2018). Studies have demonstrated a lack of correlation between devices (Dix et al, 2013, Hessel et al, 2014, Tomlin et al, 2017 and Schmidt et al, 2018), possibly due to differences in the sensor technology, wavelengths and algorithms and thus confounds interpretation of study results and indicates that devices cannot be used interchangeably. One study in children undergoing CPB surgery found that INVOS correlated more strongly than Foresight with jugular bulb SvO2 at SVC cannulation (Naguib et al, 2017).
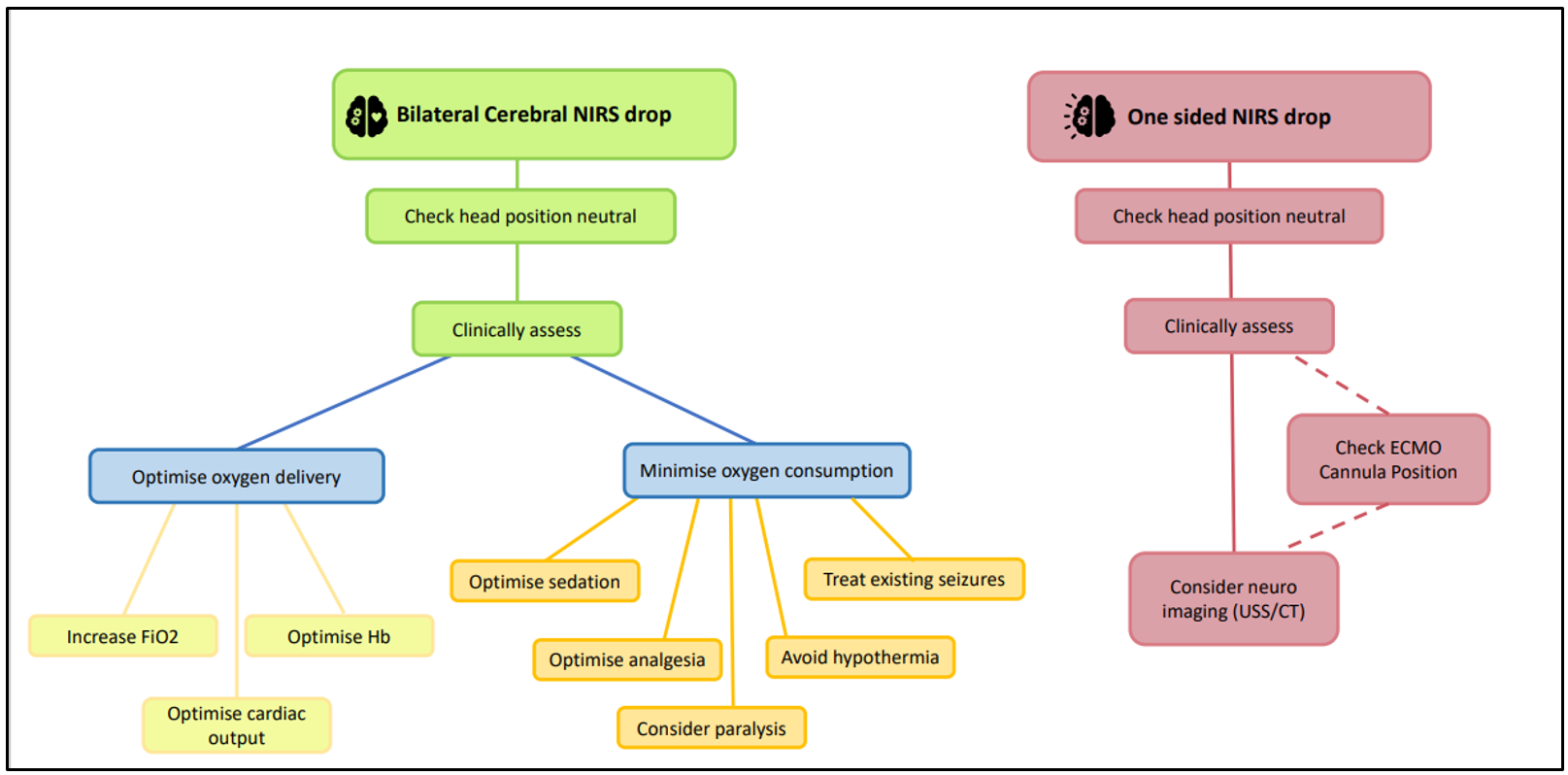
Bilateral Cerebral NIRS
An increasing number of studies are demonstrating the ability of cerebral oximetry monitoring to detect clinically silent episodes of cerebral ischaemia, highlighting the need for monitoring to be bilateral (Gottleib et al, 2006). It should be remembered that NIRS probes will only detect changes in tissue oxygen saturation in the areas local to the probes eg superficial frontal lobes, and not those in other areas of the brain. Patients undergoing cardiac surgery are particularly at risk of adverse peri-operative neurological events and use of bilateral cerebral oximetry monitoring may enable early detection of these events. (Tosh and Petteril, 2016). Such events may be due to: the absence of an intact Circle of Willis, instrumentation of the internal jugular veins, cardio-pulmonary bypass cannulas, aortic cross-clamping and periods impaired blood flow, which may be due to poor venous drainage from the brain, and therefore bilateral cerebral NIRs may detect alterations in perfusion between the different sides of the brain and enable appropriate corrective action to be taken.
Renal NIRS
AKI is a common problem encountered in critically ill children (Kavaz et al, 2012), with neonates being particularly vulnerable (Kriplani et al, 2016). AKI has been reported in up to 64% of infants following cardiac surgery. Those most severely affected require renal replacement therapy and is associated with increased; length of stay, ventilation days and mortality (Ruf et al, 2015, Morgan et al, 2013, Ayden et al, 2012, Blinder et al, 2012 and Li et al, 2011).
Following corrective surgery in infants with cyanotic cardiac defects it has been demonstrated that recovery of renal vasculature may be delayed for up to 48 hours, thereby indicating a period of vulnerability to renal dysfunction (Ersoy et al. 2016). Renal NIRs values (rSO2) have been found to strongly correlate with venous oxygen saturation from the renal vein and inferior vena cava in children weighing ≤ 10kg with congenital heart disease undergoing cardiac catheterisation (Ortmann et al, 2011). Furthermore, a decline in renal NIRS (rSO2) during cardiac surgery correlates with the development of Acute Kidney Injury and may be superior to conventional biochemistry markers (Ruf et al, 2015). This was supported by a further study conducted by Adams et al (2019) which identified that infants who developed AKI had lower mean rSO2 values in the initial 48hours post-operatively and may not be identified by monitoring creatinine alone.
Splanchnic NIRS (Gut NIRS)
Splanchnic (Gut) NIRS is not routinely used in clinical practice, however, a prospective study involving 50 infants with congenital heart disease identified that 18% experienced gastrointestinal complications in the immediate post-operative period, of which 6% had confirmed Necrotising Enterocolitis (NEC) and 3% suspected NEC (Iliopoulos et al, 2015). It has been reported that 1.62-7.8% of infants following cardiac surgery develop NEC (Mukherjee et al, 2010, Bergounioux et al 2005 and McElhinney et al, 2000) and tissue hypoxia is thought to be central to the pathophysiology (Stapleton et al, 2008).
Studies have demonstrated that mesenteric rSO2 obtained using NIRS correlates with the development of gastrointestinal complications including NEC (Fortune et al, 2001, DeWitt et al, 2014, Iliopoulos et al, 2015 and Schat et al 2016).
- Infants less than 6 months of age
- Any patient undergoing left heart bypass (Bilateral Cerebral and Renal NIRS)
- Any patient managed on ECLS
- At the discretion of the Consultant Anaesthetist
All patients may be monitored with bilateral cerebral NIRS intra-operatively and renal monitoring at the discretion in the Consultant Anaesthetist. Please note that consideration is needed regarding the siting of diathermy pad which must not be in contact with renal NIRS probe.
Consideration may be given to commencing NIRS monitoring in the anaesthetic room.
NIRS should be used in all cardiac patients who are:
- Infants less than 6 months of age with either:
- Low cardiac output state or potential for it to develop (eg post-cardiopulmonary bypass cases, Coarctation repair, etc)
- Cyanotic congenital heart disease patients with a ‘balanced’ circulation.
- Pre-operative ductal dependent lesions (i.e. prostin dependent)
- Requiring respiratory +/- vasoactive agent(s).
- Any patient with Cardiomyopathy or Myocarditis
- Any patient supported on ECLS (please refer to ECLS manual)
NB Renal NIRS is not routinely used for those < 6months of age.
At the discretion of the Consultant Intensivist NIRS may also be considered in:
- Severe sepsis
- Trauma and Traumatic Brain Injury
- Severe limb injury / fasciotomies (please refer to ECLS manual)
Triple site monitoring (bilateral cerebral and renal rSO2) is used in PICU because this not only provides continual assessment of regional venous saturations of both the brain and below the diaphragm but also provides an indication of the adequacy of total cardiac output.
Little evidence is available regarding the ‘normal’ NIRS values for children and this is confounded in the neonatal period due to alterations in physiology of the newborn (Harer and Chock, 2020) and also if CHD is present. However, some suggested thresholds are outlined below:
- Cerebral NIRS value (rSO2) < 60% could be viewed as abnormal
- Cerebral NIRS value (rSO2) <40% is abnormally low
Cerebral rSO2 <40% is associated with neurological morbidity and requires urgent review - A difference of >10% between cerebral and renal is significant (Hoffman et al, 2004).
- A decrease of 20% in any NIRS measurement
- A difference of 10-20% between right and left cerebral NIRS (rSO2) values – only minimal difference should be observed
- Cerebral values of >95% may indicate lack of O2 extraction indicated potential cerebral injury
- In those with CHD:
- Acyanotic (usual) circulation
- Arterial sats >95%
- SvO2 >65%
- Cerebral NIRS >55%
- Renal NIRS >65%
- Cyanotic circulation (more at risk of ischaemia and hypoxia because of the reduced oxygen supply capabilities).
- Arterial sats ~ 75-80%
- SvO2 45-50%
- Cerebral NIRS >40%
- Renal NIRS >50%
- Acyanotic (usual) circulation
A reduction in NIRS value (rSO2) can be due to:
- Inadequate tissue perfusion due to a reduction in O2 delivery
- Excessive oxygen consumptionin the tissues
The trend in value is as important as the actual NIRS (rSO2) value
In older children cerebral rSO2 values will be slightly lower than renal rSO2 (Ozturk et al, 2020). This is reflective of the combination of both a higher cardiac output delivered to the renal bed and lower oxygen extraction by the kidneys, compared to the brain.
- If the NIRS value (rSO2) are causing concern:
- Check NIRS probe position and adherence (The signal strength indicator should display 5 green bars if optimal detection is being generated)
- Obtain arterial and patient mixed venous blood gases and note the AVO2 differential.
- Inform a senior clinician.
- Consider trend of rSO2 in addition to actual value displayed.
- Review the patient clinically, including the assessment of late clinical markers of cardiac output i.e. lactate, peripheral-core temperature gap, urine output and GCS.
Clinical Scenarios
1. ⇩Cerebral NIRS and ⇩Renal NIRS
- Indicative of reduction in global oxygen delivery or global increase in oxygen consumption
Confirm with paired arterial and venous blood gas. Note SvO2 differential and lactate, although these are late signs of poor tissue perfusion.
- ? ⇧ oxygen consumption suspected, treat by:
- analgesia, sedation, cooling, paralysis
- ? ⇩ oxygen delivery suspected, treat by:
- Consider whether the arterial blood is optimally saturated (care in patients with parallel circulations as the ventilation changes may adversely upset the ‘balance’ - recruitment manoeuvres, ⇧PEEP, ⇧PIP, ⇧ FiO2).
- Augmentation (inotropes) and/or manipulation (vasoactive agents) of cardiac output.
- Red cell transfusion (DO2 = CO x (1.39 x Hb) x SaO2 + (0.003 x PaO2)).
- Additional considerations:
- Observe urine output closely and creatinine due to risk of AKI development.
- Pause enteral feeds – due to concern of gut ischaemia and consider abdominal CXR to assess for radiological evidence of NEC.
- If on ECLS:
- VA-ECMO = diagnostic oxygenator membrane checks + check optimal circulating blood flow.
- VV-ECMO = diagnostic oxygenator membrane checks + exclude evidence of recirculation + exclude evidence of right heart failure and the need for inotropic support.
2. ⇩ Cerebral NIRS (cranial blood supply cannot meet demand = risk of neurological injury)
- Consider thrombo-embolic event / intra-cranial haemorrhage - neurological imaging may be a useful adjunct.
- ? ⇧ oxygen consumption, suspect if:
- Pain, agitation awake, hyperthermia, seizure (including subclinical seizures)
- ? ⇩ oxygen delivery,
- ⇩PaCO2 = powerful cerebro-vascular vasoconstrictor. (Excessive ventilation; if on ECMO, consider reducing sweep gas flow).
- If on ECMO - check arterial cannula position
- If on ECMO with neck cannula – change head position to improve cerebral blood flow)
3. ⇩ Renal NIRS (infra-diaphragmatic blood supply cannot meet demand = ↑risk of renal or gut ischaemia / infarction).
- ? ⇩ oxygen delivery,
- If on ECMO - check cannula position
- Secondary to systemic vasoconstriction (vasopressin/nor-adrenaline)
- Secondary physiological compensation to reduced cerebral blood flow (Treatment strategy = see 1 above)
- Observe urine output closely and creatinine due to risk of AKI development.
- Pause enteral feeds – due to concern of gut ischaemia and consider abdominal CXR to assess for radiological evidence of NEC.
Note: ⇩renal NIRS may reflect the presence of a PDA – lower infra-diaphragmatic arterial saturations resulting in lower renal tissue saturations. This occurs in clinically significant PDAs due to ‘steal phenomenon’ from the distal systemic circulation which jeopardises the adequacy of oxygen delivery to the renal tissue.