Prescribing of triple therapy
Consider triple therapy for patients with COPD who are experiencing exacerbations or for those with COPD with a background of asthma and still experiencing symptoms or exacerbations.
Triple therapy has been shown to improve lung function and patient related outcomes as well as reduce exacerbations compared with LABA alone, LABA/LAMA and LABA/ICS inhalers.47 Triple therapy may be suitable for patients with COPD who are experiencing exacerbations or for those with COPD with a background of asthma and still experiencing symptoms or exacerbations. Use of triple therapy inhalers should increase adherence by patients, is cost effective and will have a reduced carbon footprint versus multiple inhalers.
Lifestyle aspects of therapy should be optimised when considering a move onto triple therapy. This includes smoking cessation as well as excluding other potential causes of breathlessness or poor control (adherence, inhaler technique). A clinical review after three months is recommended in order to assess benefit from the triple therapy, discontinuing the ICS if there is no improvement.46 This review can be completed in primary or secondary care.
The current triple inhalers Trelegy® Ellipta®, Trimbow® (MDI and Nexthaler®), Trixeo® Aerosphere® and Enerzair®Breezhaler® have differences in licensed indication55-60 outlined in the table below. See individual Summary of Product Characteristic (SPC) for licence details. The information in the table below was correct at the time of publication.
Licensed indications of triple inhaler
| Triple inhaler | Licensed for asthma | Licensed for COPD |
| Enerzair® Breezhaler® (indacaterol 114 micrograms / glycopyrronium 46 micrograms / mometasone 136 micrograms)55 | Yes | No |
| Trelegy® Ellipta® (fluticasone 92 micrograms / umeclidinium 55 micrograms / vilanterol 22 micrograms56 | No | Yes |
| Trimbow® pMDI (beclomethasone 87 micrograms / formoterol 5 micrograms / glycopyrronium 9 micrograms)57 | Yes | Yes |
| Trimbow® (beclomethasone 172 pMDI micrograms / formoterol 5 micrograms / glycopyrronium 9 micrograms)58 | Yes | No |
| Trimbow® NEXThaler® (beclomethasone 88 micrograms / formoterol 5 micrograms / glycopyrronium 9 micrograms)59 | No | Yes |
| Trixeo® Aerosphere® (formoterol 5 micrograms / glycopyrronium 7.2 micrograms budesonide 160 micrograms60 | No | Yes |
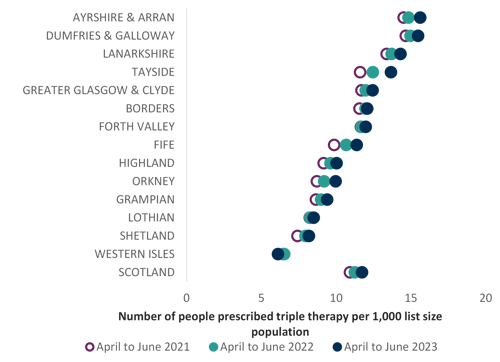
The chart below illustrates the use of triple therapy prescribing, as either separate inhalers or as a single triple inhaler, showing wide variation between NHS Boards, ranging from 15.63 in Ayrshire & Arran to 6.13 in the Western Isles. This may vary with prevalence of COPD within board regions.
Number of people receiving triple therapy (as single inhaler or separate inhalers)
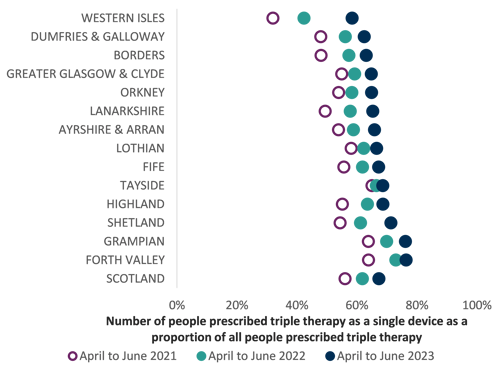
The chart below shows the individual inhaler prescribing per Health Board which potentially could be prescribed as a combination inhaler (noting that there may not be an exact equivalent available as a triple therapy inhaler).
Number of people prescribed triple therapy as a single device as a proportion of all patients prescribed triple therapy
The numerical data for NTI graphs can also be viewed here.
The most up to date national therapeutic indicator data is available here.