Apixaban in DVT / PE in NHS Borders – treatment protocol

1. Drug: Apixaban 2.5mg and 5mg tablets
2. NHS Borders formulary indication: Treatment of deep vein thrombosis (DVT), treatment of pulmonary embolism (PE), prophylaxis of recurrent DVT, prophylaxis of recurrent PE in adults.
3. Dose :
- Treatment of DVT; treatment of PE: 10 mg twice daily for the first 7 days followed by 5 mg twice daily;
- Prevention of recurrent DVT and PE: 2.5 mg twice daily. When prevention of recurrent DVT and PE is indicated, the 2.5 mg twice daily dose should be initiated following completion of 6 months of treatment with Apixaban 5 mg twice daily (or with another anticoagulant).
Use with caution if creatinine clearance is 15-29ml/minute; avoid if creatinine clearance is <15ml/minute.
(Warfarin is anticoagulant of choice for this patient group).
4 Duration of treatment
- Duration of treatment/clinic review date should be included on eDL.
- 6 months treatment unless
- small below knee DVT, especially if induced (trauma, surgery etc)- 3 months treatment.
- unprovoked proximal DVT or PE - a minimum of 6 months treatment and consider long term treatment if spontaneous event (calculate DASH score - see below) and assess bleeding risk. d-Dimer to be checked 2-4 weeks after stopping treatment (sample in a green tube + request form to lab within 4 hours of being taken. If this is not possible, then patient can be sent to phlebotomy in OPD for sample).
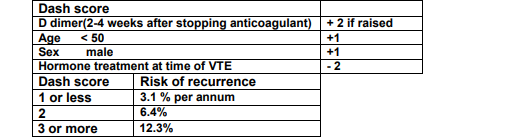
- Dash score > or = 2 consider long term anticoagulation.
- Dash score < 2, d-dimer should be checked at least 2 weeks after stopping apixaban. If d-dimer is persistently raised, patient will have a high DASH score and further investigation for underlying illness should be pursued and long term prophylaxis with apixaban started.
- High d-dimer may reflect underlying systemic illness or malignancy so should prompt review of patient and investigation if appropriate.
- Risk of significant bleeding on apixaban prophylaxis = 2 %.
5 Cautions & contraindications
- All patients being considered for apixaban should be assessed for “bleeding risk”, including PMH of upper gastrointestinal haemorrhage or upper GI symptoms, and if risk is deemed to be high, alternative anticoagulation should be considered.
- When apixaban is commenced the need for concomitant aspirin, clopidogrel, NSAIDS or other antiplatelet agents / anti-coagulants should be reviewed by consultant/senior medical staff, and stopped where possible. Borders Formulary.
- For patients at risk of ulcerative gastrointestinal disease prophylactic treatment with an oral PPI should be considered.
- Apixaban should be discontinued
- 24 hours prior to minor procedure
- 48 hours prior to major procedure
- 48 hours prior to lumbar puncture
- If removing an indwelling epidural catheter (post-op analgesia) wait for 20-30 hours after apixaban dose, and do not give next dose of apixaban until at least 5 hours after catheter removal.
If procedure cannot be delayed, consider the increased risk of bleeding v the urgency of
intervention.
Apixaban should be restarted as soon as possible provided the clinical situation allows and adequate haemostasis has been established – usually 6-12 hours later or as advised by surgeon.
- Apixaban is not recommended during the acute phase for patients with pulmonary embolism who are haemodynamically unstable or may receive thrombolysis or pulmonary embolectomy (safety and efficacy of apixaban have not been established in these clinical situations).
- Refer to product information for contraindications and further information.
6. Supply
- On discharge from BGH, the patient receives a 21 day supply of apixaban with dose instructions for: 10mg.
7. Drug Interactions
- Apixaban should not be co-prescribed with strong inhibitors of both CYP3A4 and P-gp, such as azole-antimycotics (e.g., ketoconazole, itraconazole, voriconazole and posaconazole) and HIV protease inhibitors (e.g., ritonavir);
- Apixaban should not be co-prescribed with strong CYP3A4 and P-gp inducers (e.g.rifampicin, phenytoin, carbamazepine, phenobarbital, St. John's Wort).
- See cautions, above, re aspirin, clopidogrel, NSAIDs, anti-platelets/anticoagulants.
- Refer to BNF & SPC for further interaction information.
8. Patient counselling.
(Clinician responsible for counselling patient must document this in patients medical notes).
- Apixaban patient alert card should be completed – addressograph added & information booklet issued.
- Ensure patient is aware of potential adverse reactions.
- Ensure that patient understands the prescribed dose to take.
9. Monitoring – patient safety:
- Patients should be advised not to take apixaban on the evening before or the morning prior to any diagnostic procedure (eg colonoscopy, endoscopy).
- Patients should be counselled on the symptoms and signs of bleeding prior to discharge from BGH and an information leaflet supplied. This is particularly important for the at risk patient groups highlighted above.
- Monitor creatinine clearance in patients with renal impairment - check creatinine clearance after 1 month and after 3 months.
10. Management of bleeding.
In the event of haemorrhagic complications, treatment must be discontinued and the source of bleeding investigated. Guidance for reversal.
11. Switching
- Switching treatment from parenteral anticoagulants to Apixaban (and vice versa) can be done at the next scheduled dose time - these agents should not be administered concurrently.
- Switching from warfarin to apixaban; discontinue warfarin and start apixaban when INR is < 2.0.
- Switching from apixaban to warfarin; continue administration of apixaban for at least 2 days after commencing warfarin. After 2 days of coadministration of apixaban and warfarin take an INR prior to the next scheduled dose of apixaban. Continue coadministration of apixaban and warfarin until the INR is ≥ 2.0.
12. Further information
- There is currently no PE/DVT clinic in NHS Borders. (Respiratory follow up patients with PE at 3 months if miller score >8).
- Patients with DVT require to wear class 2 below knee stockings for 2 years. Podiatry should be contacted to supply for BGH inpatients . Patients admitted acutely should be issued, by discharging doctor, with an HBP prescription for 2 pairs of class 2 below knee stockings to take to community pharmacy for measurement and supply.