| Drug name | Method of administration for NG tubes or swallowing difficulties |
| Amantadine | Omit |
| Co-beneldopa (including MR) | Change to dispersible preparation (same dose) |
| Co-careldopa (including MR) | Change to co-beneldopa dispersible (same dose) |
| Entacapone | Omit |
| Tolcapone | Omit |
| Co-careldopa +Entacapone | Change co-beneldopa dispersible (same dose) Omit entacapone |
| Pramipexole (including MR) | Use rotigotine patch (refer to conversion table 2) |
| Ropinirole (including XL) | Use rotigotine patch (refer to conversion table 2) |
| Rasagiline | Omit |
| Selegiline | Omit |
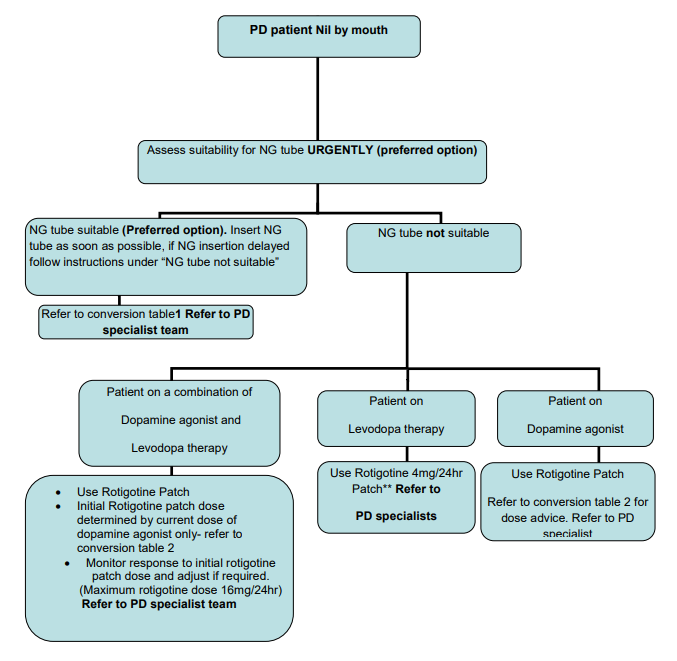
Parkinson’s disease patients in the acute setting (with swallowing difficulties or who are nil by mouth; including peri-operative)

NHS Borders Guidance for Parkinson’s Disease patients in the acute setting with swallowing difficulties or who are nil by mouth (including peri-operative).
- The Parkinson’s disease specialist team are the point of contact for expert advice during normal hours.
- Out of hours the guidance below should support safe prescribing and symptom control.
- With all changes to PD medication, close monitoring of the patient is indicated.

| Pramipexole - base content | Prolonged Release Pramipexole- base content | Ropinirole | Modified Release Ropinirole | Rotigotine Patch |
| 0.088mg tds (0.125mg tds salt content) | 0.26mg/day (0.375mg/day salt content) | Starter pack | N/A | 2mg/24hrs |
| 0.18mg tds (0.25mg tds salt content) | 0.52mg/day (0.75mg/day salt content) | 1mg tds | 4mg/day | 4mg/24hrs |
| 0.35mg tds (0.5mg tds salt content) | 1.05mg/day (1.5mg/day salt content) | 2mg tds | 6mg/day | 6mg/24hrs |
| 0.53mg tds (0.75mg tds salt content) | 1.57mg/day (2.25mg/day salt content) | 3 mg tds | 8mg/day | 8mg/24hrs |
| 0.7mg tds (1mg tds salt content) | 2.1mg/day (3mg/day salt content) | 4mg tds | 12mg/day | 10-12mg/24hrs |
| 0.88mg tds (1.25mg tds salt content) | 2.62mg/day (3.75mg/day salt content | 6mg tds | 16mg/day | 14mg/24hrs |
| 1.05mg tds (1.5mg tds salt content) | 3.15mg/day (4.5mg/day salt content | 8mg tds | 24mg/day | 16mg/24hrs |
- ** Guidance differs from manufacturers license.
- Frail elderly patients are particularly prone to the side-effects of rotigotine patches.
- If possible avoid use of rotigotine patches in patients with vascular parkinsonism – levodopa preparations preferred.
- Area for application of rotigotine patch should be rotated; patches should be held in place for 30 seconds to ensure adherence to skin.
- If patient is NBM & has had a reaction to Rotigotine patch, contact ERI (01315371000) and ask for the on call medical neurology registrar.
- Monitor patients for side-effects/lack of benefit.
- Adhere to normal dose times when switching to dispersible co-benelopa preparation.
- When period of swallowing problems/nil by mouth resolves, switch PD patient back to normal medication regime.
- All medications contained in this guidance are available in BGH emergency drug cupboard (access available from nursing bleep holder).
- Place PD patients first on operating list.
- Review dosing regimen: If timing of PD medication is going to clash with surgery the regimen needs to be altered – discuss with PD Nurse Specialist for advice regarding alteration of timings.
- Review regular medication prior to surgery and ensure morning doses of all PD medications are prescribed and administered (drug chart should be clearly marked to indicate that they must be given prior to surgery).
- If the total duration of surgery or NBM status is expected to be >6 hours please contact PD Nurse Specialist. Rotigotine transdermal patch may need to be considered for this period.
- Deep Brain Stimulation: ensure surgeon is aware of this as diathermy will be contra-indicated.
Jill Anderson PD nurse specialist Jill.Anderson@borders.scot.nhs.uk (working hours are Monday 8am-6pm, Tuesday 8am-5pm, Wednesday 8am-4.45pm. Telephone 01896827025; mobile 07500917130.
Dr Myles Connor Consultant Neurologist Myles.Connor@borders.scot.nhs.uk
Dr David Simpson Consultant Neurologist David.Simpson@borders.scot.nhs.uk
Dr Andrew McLaren Consultant Physican Andrew.McLaren@borders.scot.nhs.uk
Dr Rachel Stewart Consultant Physican Rachel.Stewart@borders.scot.nhs.uk
Thanks to NHS GG & C for permission to use information from their guideline in development of this guidance.
Originally developed by NHS Borders PD Formulary Group May 2017; reviewed February 2020 and 2022 (no changes required). For Review February 2024