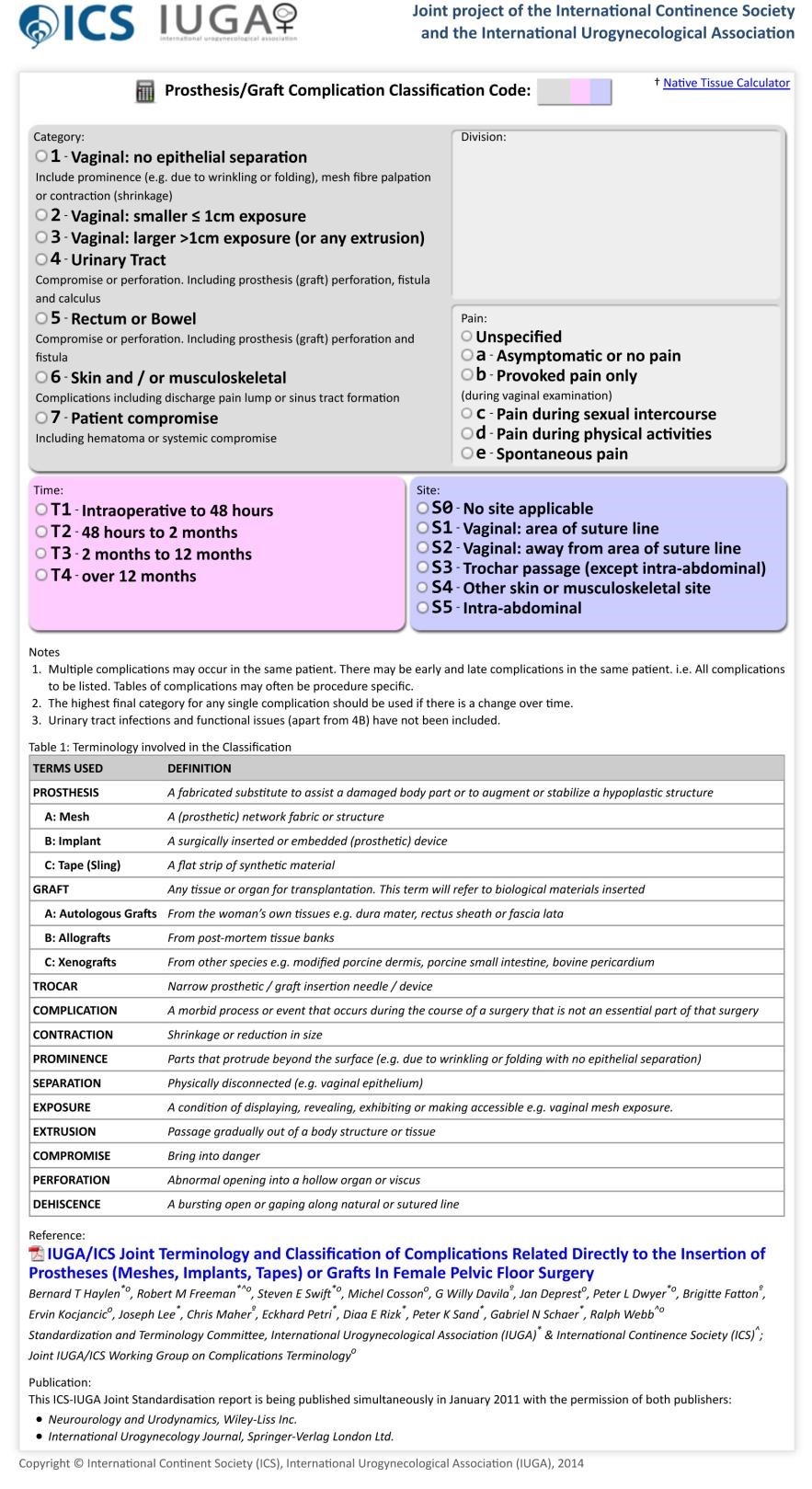
- Complications should be classified according to the International Urogynaecological Association (IUGA) and International Continence Society (ICS) classification system. (Appendix A)
- Original surgeon (if different from referring clinician) must be informed of complication to enable them to maintain an accurate audit and records.
- Reporting of complications to either, NHS Scotland Incident Reporting and Investigation Centre (IRIC) or Medicines & Healthcare products Regulatory Agency (MHRA) is mandatory. This should be done by inserting consultant, but if unavailable, by diagnosing clinician.
- Datix completion for procedures performed in Greater Glasgow and Clyde (GGC)
- Inform lead clinician of complication for audit and clinical governance purposes.
Pathway for women presenting with graft complications in Urogynaecology

Objectives
To outline the referral pathway and points of management for women with graft complications relating to urogynaecology including those with mid urethral tapes/continence procedures or prolapse meshes (any compartment)
Audience
All healthcare professionals who care for women where a graft complication is suspected
Please report any inaccuracies or issues with this guideline using our online form
This guideline includes information regarding the referral pathway and management of graft complications including women with mid urethral tapes/ continence procedures or prolapse meshes (any compartment).
Women with previous tapes and/ or prolapse meshes require a diagnostic flexible cystoscopy to exclude graft-related complications where they present with symptoms of :
- Recurrent UTIs
- Refractory overactive bladder symptoms
- Bladder / urethral pain
- Voiding dysfunction
Where mesh removal is required or requested, the referral pathway is outlined in the GGC guideline ‘Specialist Mesh Removal Referral Process’. The referral form can be found within the guidelines Mesh Removal referral pathway.
All women undergoing mesh removal (partial or complete) must be discussed at the GGC Urogynaecology Multi-Disciplinary Team (MDT) meeting.
Specimens must be managed in accordance to agreed Scottish Central Legal Office (CLO) protocol. The details are outlined in (Appendix B)
All patients undergoing mesh removal have either:
- Commenced medico-legal proceedings relating to the mesh implant
- May in due course commence medico-legal proceedings relating to the mesh implant
The explanted mesh specimen remains evidence for these medico-legal actions.
The following guidelines for management of explanted mesh within Women & Children’s Directorate in GGC have been drawn up with National Services team Scotland Central Legal Office.
Management:
- Medical Photography
- All patients to complete GGC Medical Illustration Consent form. If a patient declines to sign consent form, they cannot have photographs taken and stored. If a patient declines photography, this must be documented in notes.
- Where consent is obtained, all cases of explanted mesh are to be photographed by medical illustration, after removal from the patient in theatre.
- The implant is to be photographed on a clean white swab with a ruler for indication of size, prior to placing all explanted implant into formaldehyde.
- Medical illustration is to be contacted by secretary/waiting list manager when patient is listed for removal of tape or mesh, thus allowing medical illustration time to provide photographer services for the theatre list.
- The need for medical photography is to be highlighted on theatre list to ensure theatre team are aware, and therefore liaise accordingly.
- The operating team are to confirm with medical illustration at pre-assessment visit.
- Consultant team to then liaise with medical illustration on day of surgery about time of procedure and when photographer will be required.
- Histology
- All samples are to be sent to histology in formaldehyde with a histopathology request form for routine processing and analysis of sample.
- Within GGC, samples are to be marked for the attention of: Colin Thatcher / Lorraine Watson at Specimen Reception, Queen Elizabeth University Hospital, Glasgow (QEUH )
- Uplift by Clinical & Evidence Storage Ltd (CES)
- CES to liaise directly with Histopathology labs for uplift of specimen if required for medicolegal proceedings