No single test offers 100% sensitivity or specificity for the detection of ovarian cancer. CA125 is elevated in >80% of epithelial ovarian cancers. However a maximum of only 50% of women with clinically detectable stage 1 disease have elevated levels. Ovarian cysts in post-menopausal women should be assessed using CA125 and trans-vaginal ultrasound which offers greater sensitivity than the trans-abdominal method. However larger cysts may require to be assessed abdominally. Routine use of other imaging techniques is not recommended (MRI /CT), although these may be of value in selected cases.
Ovarian Cysts in Post-Menopausal Women (549)

Please report any inaccuracies or issues with this guideline using our online form
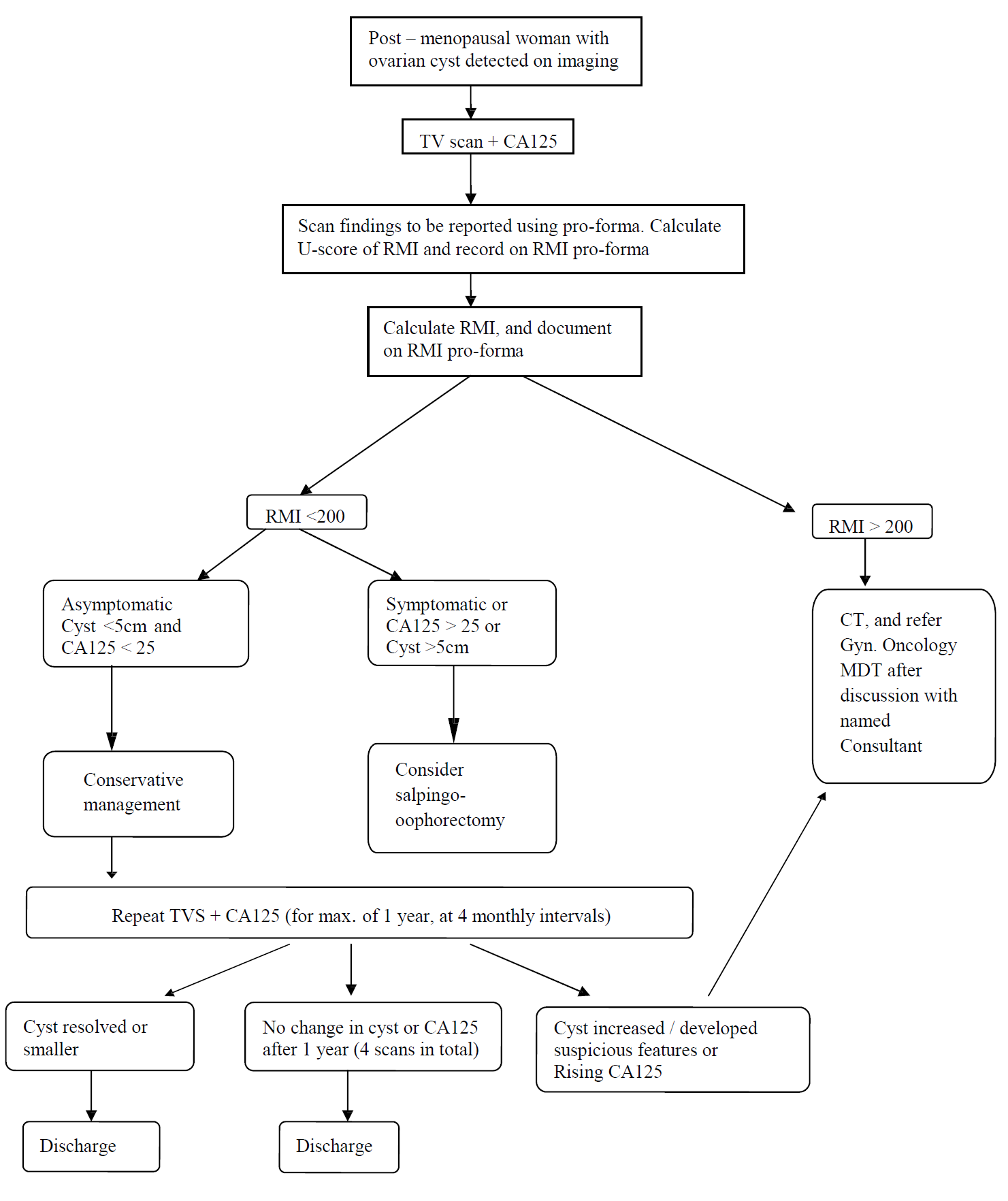
Ovarian cysts are common in post-menopausal women (amenorrhoea for 1 year or more), and may be discovered during investigation of gynaecological symptoms or during imaging for other reasons.
Many of these cysts will have a low risk of malignancy, and not all need to be managed surgically. However it is important to triage women appropriately to decide the correct management and place for this to occur.
The use of RMI scoring has been shown to be an effective method of determining which women are at low / medium/ high risk of malignancy. RMI scoring includes measurement of CA125 and the assessment of specific ultrasound features. Therefore, ultrasound reporting must detail the morphological features present to enable calculation of the RMI accurately.
The RMI is calculated as follows:
RMI = U x M x CA125
U depends on presence of the following ultrasound features:
- Multi-loculation
- Evidence of solid areas
- Evidence of metastases
- Bilateral lesions
- Presence of ascites
U value 0: no ultrasound features
U value 1: 1 ultrasound feature
U value 3: 2 – 5 ultrasound features
M scores 3 for post-menopausal women (1 for pre-menopausal)
See attached pro-forma for Calculation of RMI
Simple unilateral, unilocular ovarian cysts measuring <5cm, in the presence of a normal CA125, should be managed conservatively in the vast majority of cases, with follow-up TV scans and CA125 measurements, which should be performed at 4-monthly intervals for a year. If there has been no change over the course of a year then the woman can be discharged.
If the RMI is <200, but the woman is symptomatic or CA125 >25, then surgical management should be considered (oophorectomy). Surgery should also be considered if the RMI is < 200 but the cyst >5cm in diameter.
If the RMI is >200, then the case should be referred to the Gyn / Oncology MDT for surgery in a cancer centre, and a CT scan of chest, abdomen and pelvis arranged in the interim.
The management is summarised in the flow diagram.
Flowchart for the management of ovarian cysts in post-menopausal woman