- As a diagnostic procedure to determine the cause of ascites and ascertain cytological diagnosis. It should be kept in mind there are other causes of ascites other than malignancy. It can be helpful to also send ascites to microbiology and biochemistry. It can be useful to have a serum to-ascites Albumin Gradient (SAAG) if the ascites turns out not to be malignant.
- As a therapeutic procedure: in patients with large volume ascites to provide symptomatic relief.
Ascitic Drain Insertion And Management in Gynaecology (1077)

Objectives
To describe the indications, procedure and monitoring required for the safe insertion of Ascitic Drains in gynaecology including post insertion monitoring
Scope
Women attending with Ascites to Gynaecology where management with Paracentesis Drain is considered
Audience
All healthcare professionals involved in the care of women with ascites who require insertion of Ascitic drain in the gynaecology setting
Please report any inaccuracies or issues with this guideline using our online form
Malignant ascites is the accumulation of fluid within the peritoneal cavity of the abdomen secondary to cancer (an exudate not a transudate). Within gynaecology, ascites is most commonly associated with those suffering with ovarian malignancy. Approximately 60% of patients with advanced ovarian cancer develop ascites necessitating hospital admission and treatment.
The symptoms associated with ascites can be distressing for the patient including abdominal distension and pain, breathlessness, nausea, vomiting, early satiety, constipation and peripheral oedema. These can greatly impact on patients’ quality of life.
The principle for management of malignant ascites should be aimed at symptomatic relief and improvement of quality of life. For most patients, paracentesis is the treatment of choice and relieves symptoms in up to 90% patients. It is most likely to relieve symptoms of abdominal distension/discomfort and dyspnoea, however less likely to improve the associated symptoms of peripheral oedema, fatigue and poor mobility.
Paracentesis is a simple procedure to remove ascitic fluid from the abdominal cavity, which can be performed as a day case or as an inpatient. Removal of 4–6 litres is usually enough to give symptomatic relief. Removal of more than 4-6 litres increases the risk of hypovolemia and adverse effects, but may give symptomatic relief for longer until the ascites re-accumulates.
Paracentesis may not be appropriate if the prognosis is very short and the patient is rapidly deteriorating.
- Severe bowel distension
- Severe coagulopathy
- Disseminated intravascular coagulation
- Local or systemic infection
- Low white cell count / neutropenia
- Skin infection at the proposed puncture site
- Distressed/uncooperative patient
The risk associated with paracentesis can be associated with the removal of large volumes (> 6 litres) as this can cause a ‘fluid shift’ with resultant hypovolaemia and hypotension, leading to symptoms of dizziness, fatigue and malaise. Additional procedural risks include; perforation of abdominal viscus, haemorrhage, infection, discomfort. See appendix 1 for risks to be discussed with patient when consenting for procedure.
Drain insertion should be performed under ultrasound guidance or following marking. Care should be taken particularly if ascitic fluid is not easily clinically identified, there has been difficulty with previous paracentesis, there is suspected loculation of ascites or there are concerns about bowel obstruction. Consideration should be given to assistance from radiology department where difficulty is anticipated.
- Review up to date FBC, coag, U+Es and LFTs
- Check IV access sited and patent
- Ensure site has been marked if USS guidance not available
- Ensure nursing staff available to assist insertion
- Obtain informed consent
- Patient should empty bladder prior to procedure
- Check full set of observations including – pulse, BP, temperature, respiratory rate and O2 saturations
- Ensure correct equipment available – see appendix 2 for required equipment
- Place patient in semi upright position
- Confirm the presence of ascites
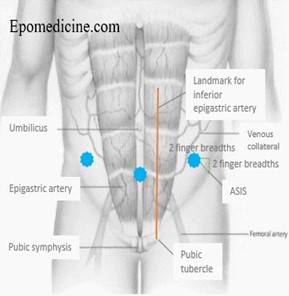
- The usual site for paracentesis is the left side but can be in either iliac fossa at least 10cm from midline or suprapubically (must ensure bladder is empty). The chosen site should avoid: distended bowel or bladder and inferior epigastric arteries. Guided by ultrasound marking if trained.
- Abdomen is prepared with aseptic solution and sterile drape applied
- Infiltrate up to 10ml of 2% lidocaine into the area to be cannulated.
- Using local anaesthetic syringe, needle is inserted through sheath and ascites withdrawn. A larger needle may need to be considered in patients with a high BMI
- When anaesthesia of insertion site achieved small incision made with scalpel and drain needle inserted. Once flashback is visualised, advance the catheter whilst withdrawing the needle.
- If cytology required minimum 200ml should be sent
- It can be useful to consider sending fluid to microbiology and biochemistry, particularly where the ascites turns out not to be malignant. Tests should include Cell count and differential, Bacterial culture, Albumin (for serum-to-ascites albumin gradient (SAAG)), Total protein, Glucose and LDH.
- Closed drainage system is attached.
- If drain is to remain in situ sutures are inserted and sterile dressing applied.
- Document the procedure, plan for drainage and required frequency of observation in the notes.
- Record full set of observations again and volume drained immediately post procedure
- Record BP, pulse and temperature every 15 minutes for the first hour after insertion, hourly for 4hrs and 4 hourly thereafter if within normal limits
- Monitor fluid balance and urea and electrolytes daily Observe paracentesis insertion site and change dressing daily.
- If the patient becomes unwell, clamp the drain, take pulse, blood pressure and temperature and seek medical advice.
- Aim to remove drain after 48hrs. If continuing to drain significant volumes discuss with medical staff regarding timing of removal
Drainage rate and amount
Most patients will tolerate the procedure well. Total amount drained will depend on the individual patient, the volume of ascites and previous experiences with paracentesis.
Patient group | Rate | Fluids |
Systolic BP >100 No relevant co-morbidites | Drain 1L every 2-4hrs Max 6L in 24hrs | Not usually required |
Systolic BP <100 prior to or during drainage Cardiovascular or renal comorbidities (at medical staff instruction) | Drain 1L every 4hrs Max 6L in 24hrs If BP drops significantly or symptoms of hypovolaemia* - stop drainage and seek medical advice | Consider fluid replacement with IV crystalloid |
*Signs and symptoms of hypovolaemia: high pulse, low BP; dizziness; increasing fatigue and malaise.
The drain should be clamped after each litre drained and removed once minimal volumes draining. If significant volumes still draining at 48hrs discuss with senior medical staff regarding keeping the drain in situ for ongoing drainage, this should be reviewed by a senior clinician every 24hrs.
For patients where chemotherapy is unlikely to be effective and who require repeated drainage, an indwelling catheter should be considered and should be discussed with the oncology team.