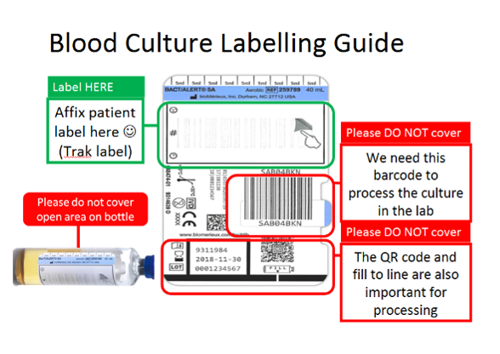
Blood cultures should always be taken when a bloodstream infection is suspected and ideally before antibiotics are given.
Clinical symptoms in a patient which may lead to a suspicion of a bloodstream infection are:
- pyrexia or hypothermia (absence of fever does not exclude blood stream infection)
- shock, chills, rigors
- severe local infections (meningitis, endocarditis, pneumonia, pyelonephritis, discitis)
- abnormally raised heart rate
- low or raised blood pressure
- raised respiratory rate