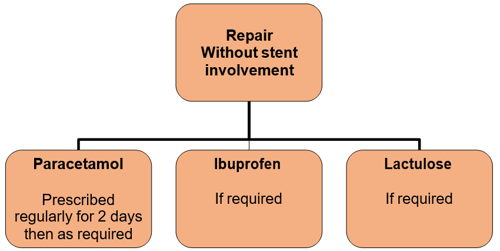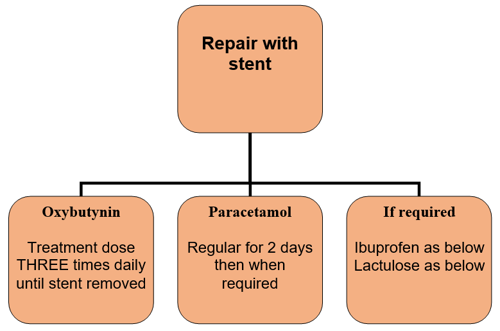
Duration of treatment is for the duration the stent remains in place. This is usually 7 days although may be ten days depending on the day surgery falls upon.
If stent is suprapubic duration of treatment may be extended as per surgical consultant’s instruction.
Use of prophylactic antibiotic post-surgery is no longer used for all patients in repair with stents but may be considered on consultant advice.