Eradication of Burkholderia Species in Children with Cystic Fibrosis, Paediatrics (700)

Disclaimer:
The following guideline has been developed for use within the Royal Hospital for Children, NHS Greater Glasgow and Clyde (NHSGGC). The guideline has been developed in collaboration with key stakeholders within NHSGGC, including Mircobiology, Cystic Fibrosis, Infectious Disease and Pharmacy teams. The guideline has been approved by the Paediatric Antimicrobial Management Team and ratified by the NHSGGC Antimicrobial Utilisation Committee.
The guideline does not account for epidemiology and resistance patterns outside of NHS GGC and use outside of the designated organisation is at the individual’s risk.
Background:
The Burkholderia cepacia complex (Bcc) consists of a group of genomic species called genomovars. Some examples include B. cepacia, B.multivorans, B. cenocepacia, B.vietnamiensis, B. stabalis, B. dolosa and B. pseudomultivorans. Reports have confirmed some strains as conferring an adverse prognosis, and colonisation with B. cenocepacia is one of the exclusion criteria from many transplant programmes.
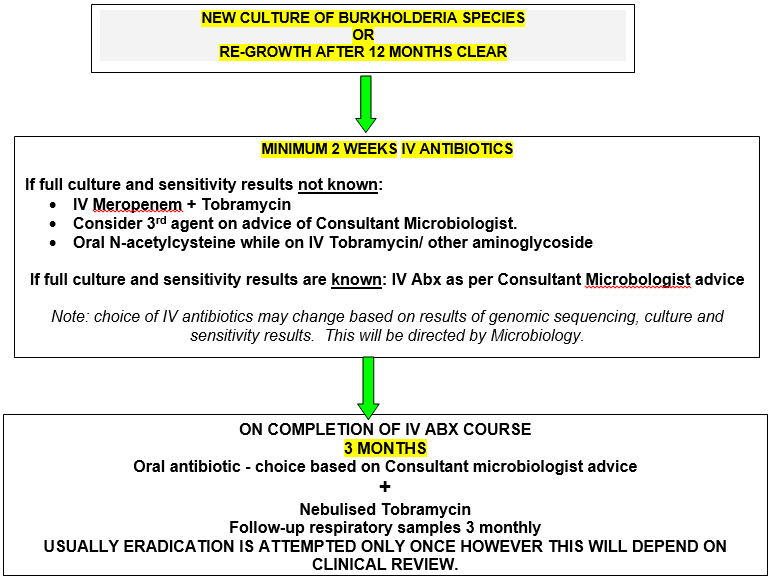
As with P. aeruginosa, some isolates of Bcc, particularly B. multivorans, can be successfully eradicated with early aggressive antibiotic therapy before chronic infection becomes established (Etherington et al, 2003).) While a COCHRANE review in 2019 did not identify any studies of sufficient quality to draw conclusions regarding specific regimes for eradication of Burkholderia and acknowledged a paucity of evidence base, the CF Trust guidelines recommend eradication attempts should be considered. The aim is to prevent chronic colonisation and subsequent impairment of lung function and is routine in many centres based on cases studies and series. Primary eradication therapy involves a combination of intravenous antibiotics. Choice is based on culture and sensitivity results (including genome typing) and this therapy is given for a minimum of two weeks, followed by three consecutive months of nebulised TOBI®.
It may be appropriate to start antibiotic therapy whilst awaiting confirmation as even if Burkholderia species are not identified, it is likely another gram negative organism (e.g Pseudomonas spp) will be identified.
Audit of this protocol shows a high level of success in eradication with currently only ~ 3.5% patients colonised with BCC. This prevalence has remained stable for many years and is in line with UK CF Registry data.
It will be important to monitor the situation as modulator drugs are more widely used and the risk benefit of antibiotics may change.
Intravenous drug dosing:
|
Drug |
Dose |
Frequency |
Comments |
|
Meropenem |
40mg/kg (max 2 grams) |
Every 8 hours |
Consider administration as a prolonged IV infusion (over 3 hours) to achieve a more favourable pK profile. |
|
Tobramycin |
10mg/kg (max 660mg) |
Once daily |
Infuse over 30 minutes. Level immediately prior to second dose.
Oral N-acetylcysteine should be prescribed with each course of IV tobramycin. |
For dosing of alternative IV agents please refer to BNF-c, or contact Microbiology or Pharmacy for advice.
Nebulised drug dosing:
|
Drug |
Dose |
Frequency |
Comments |
|
Tobramycin |
≥6 months – 2 years: 150mg ≥ 2 years: 300mg |
Twice daily |
Caution when switching between TOBI/Tymbrineb and Bramitob as solutions are different strengths. Use in children <6years is unlicensed. |
Alternative agents for nebulisation include aztreonam lysine, ceftazidime, meropenem and temocillin – Consultant Microbiology approval required. For dosing advice following approval please contact Pharmacy. Burkholderia cepacia complex spp are intrinsically resistant to colistin.
If A1555G genetic testing has not been carried out, parents/ patients should be provided with information about this and if consent obtained, blood should be sent for analysis. If the A1555G mutation is identified, the risks and benefits of continuing aminoglycoside treatment will be discussed and, where possible, alternative treatments will be considered.
ANNUAL AUDIT OF:
- BURKHOLDERIA ACQUISITION RATE
- BURKHOLDERIA ERADICATION RATE
- ONGOING ASSESSMENT OF SERIOUS DRUG RELATED SIDE EFFECTS