Allergic bronchopulmonary aspergillosis (ABPA) is a complex respiratory disorder characterised by a hypersensitivity reaction to Aspergillus fumigatus. It affects approximately 10% of patients with cystic fibrosis (CF). Untreated, inflammation may result in bronchiectasis, fibrosis and eventually loss of lung function. ABPA can be difficult to diagnose. Diagnostic criteria require an evaluation of clinical and radiological signs, lung function trend and serum immunologic markers such as total Ig E , Aspergillus IgE and Aspergillus IgG.
DIAGNOSIS
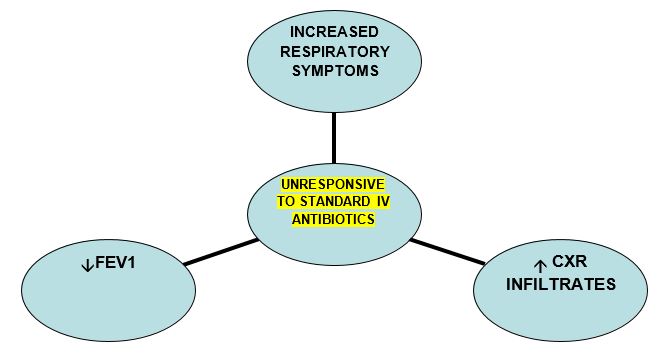
Early diagnosis and treatment are important in preventing the complications associated with ABPA. Early diagnosis depends on a combination of screening and high clinical suspicion. Atypical cases may lack some or all of the diagnostic criteria – maintain a high index of suspicion an discuss with the Consultant.
Bloods – Positive ABPA serology plus at least one other marker suggestive of ABPA
- Total IgE > 450 kU/L
- Aspergillus. Specific IgE > 0.35 kU/L
- Aspergillus. Specific IgG > 90 mgA/L
- Eosinophils > 0.4 (x109g/L)
Sputum / Cough Swabs
Aspergillus fumigatus may or may not be present in respiratory cultures.
Clinical features for consideration
- Clinical signs consistent with pulmonary infection, particularly if failing to respond to antibiotics and inhaled medications
- Fever/malaise
- Change in pulmonary function/reduction in FEV1
Other investigations
- Chest X-ray may be of use. Evidence of pulmonary infiltrates, segmental collapse or central bronchiectasis may be indicative of ABPA.
ABPA - TREATMENT
Treatment of ABPA includes a combination of oral Prednisolone plus an oralanti-fungal agent.
CORTICOSTEROIDS
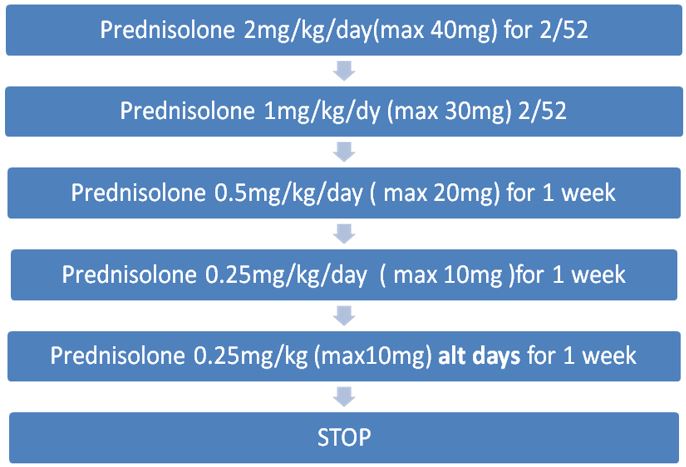
Oral corticosteroids are given to reduce inflammation and suppress immune hyper-reactivity. Use is based largely on clinical experience due to a lack of randomised controlled trials. The following dosing regimen is based on consensus from the Cystic Fibrosis Foundation.
At RHC, prednisolone is the oral steroid of choice for ABPA.
Oral prednisolone should be given in the morning after food. Enteric coated preparations should be avoided as these are not well absorbed in CF.
Varicella antibody (IgG) should be checked prior to starting steroids for ABPA. If negative, caution patient/parent to be vigilant for possible chicken pox exposure during steroid treatment.
ANTIFUNGAL TREATMENT
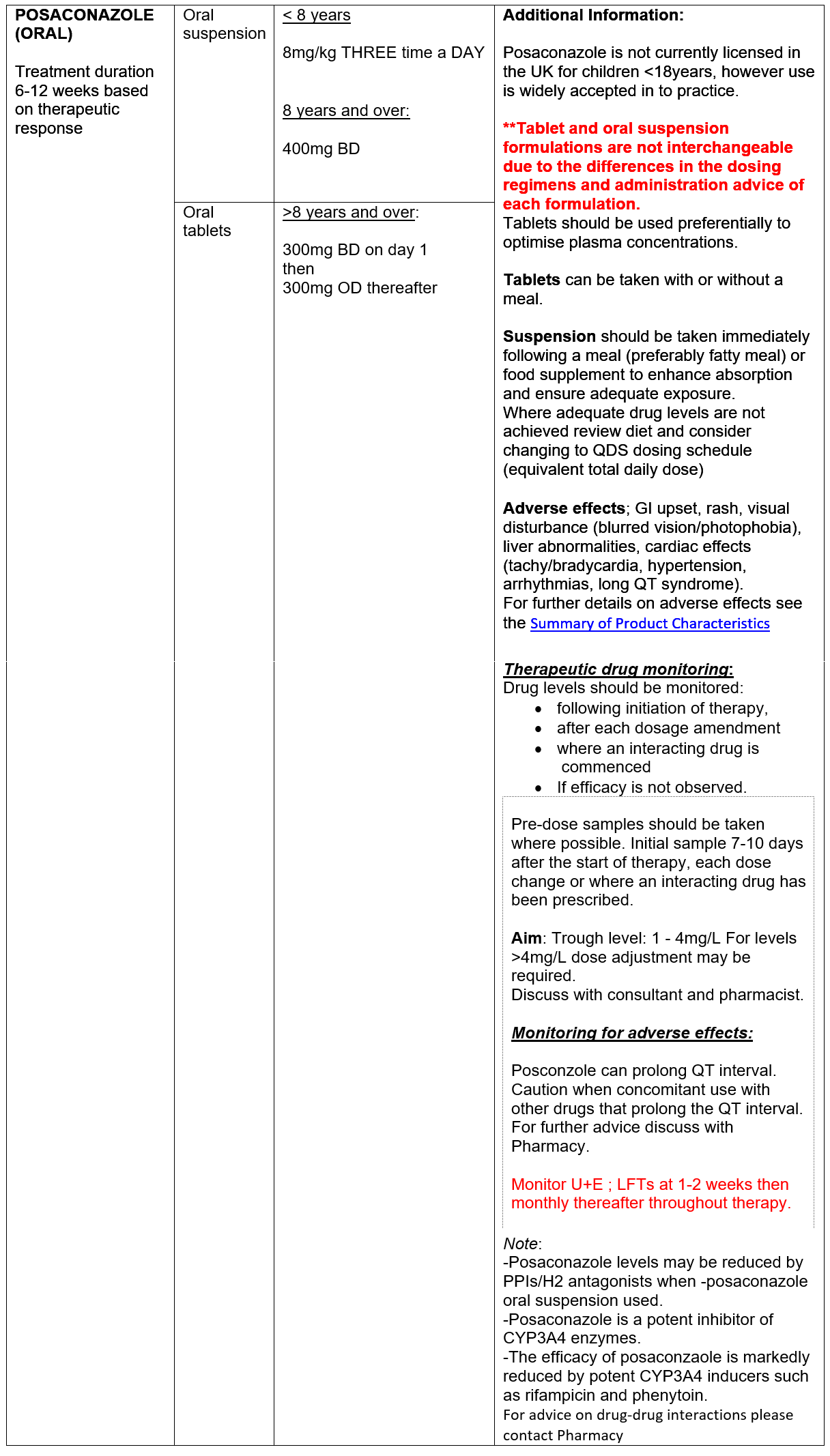
Oral ‘azole’ antifungals (AFs) are used to reduce fungal burden in the airways to attenuate immune response. Increasing data supporting the use of the ‘newer’ antifungal agents, posaconzole and isavuconazole, in CF patient with ABPA is emerging. Neither agent is currently licensed for use in children <18 years in the UK, however use has been widely accepted in to clinical practice. Posaconazole and isavuconazole have favourable side effect profiles compared to voriconazole. In addition, the newer agents have a more predictable kinetic profiles with less inter-patient variability compared with voriconazole, better facilitating therapeutic monitoring and management of drug-drug interactions.
Azole antifungal agents inhibit CYP3A4 enzymes pathways with varying degrees of potency, resulting in increased exposure to CYP3A4 substrates including Ivacaftor (Kalydeco), tezacaftor/ivacaftor (Symkevi) and elexacaftor/tezacaftor/ivacaftor (Kaftrio). Dose adjustment of the CFTR modulators is required. Interactions with lumacaftor/ivacaftor (Orkambi) are more complex - contact Pharmacy for further advice.
Failure to respond to initial therapy:
Where clinical response is poor,
- Consider IV methylprednisolone in place of oral steroids – discuss with Consultant and Pharmacy.
- Check serum azole level is therapeutic. If not review adherence and consider dose increase before switching treatment.
- Check for any newly initiated therapy and potential drug-drug interactions.
- Consider switch in antifungal therapy, taking note of any sensitivities available. Discuss with Microbiology and Pharmacy for dosing advice and in to review drug-drug interactions.
Relapse/hard to treat ABPA/frequently relapsing ABPA
Relapse is relatively common and can occur months to years after the first episode. Repeat treatment courses will be required. Changes from initial therapy should be considered – discuss with Microbiology.
All cases of relapse or difficult to treat ABPA should be discussed with the CF MDT, including Microbiology. Alternative treatment strategies may be considered, including:
- Nebulised amphotericin (non-liposomal) for up to 3 months
- Isavuconazole
- IV caspofungin
- Omalizumab – requires ULM application.
Use of voriconazole is should be avoided where possible due to variable kinetic profiles (DDIs) and limiting side effects. The MHRA published a drug safety update highlighting the risk of liver toxicity and squamous cell carcinoma following phototoxic reactions. Where voriconazole use is unavoidable, MDT discussion is required.
