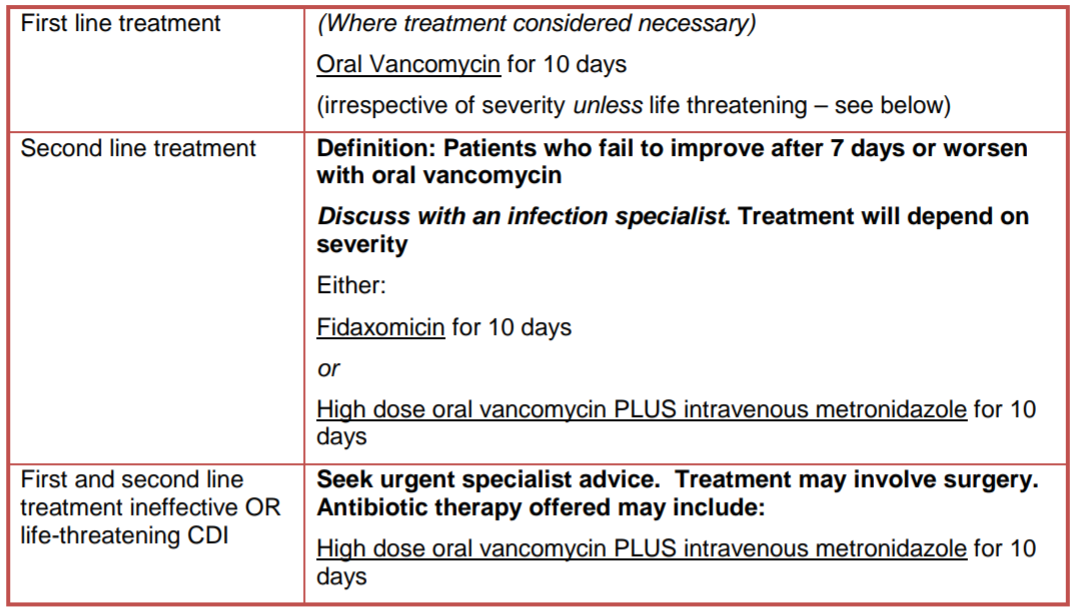
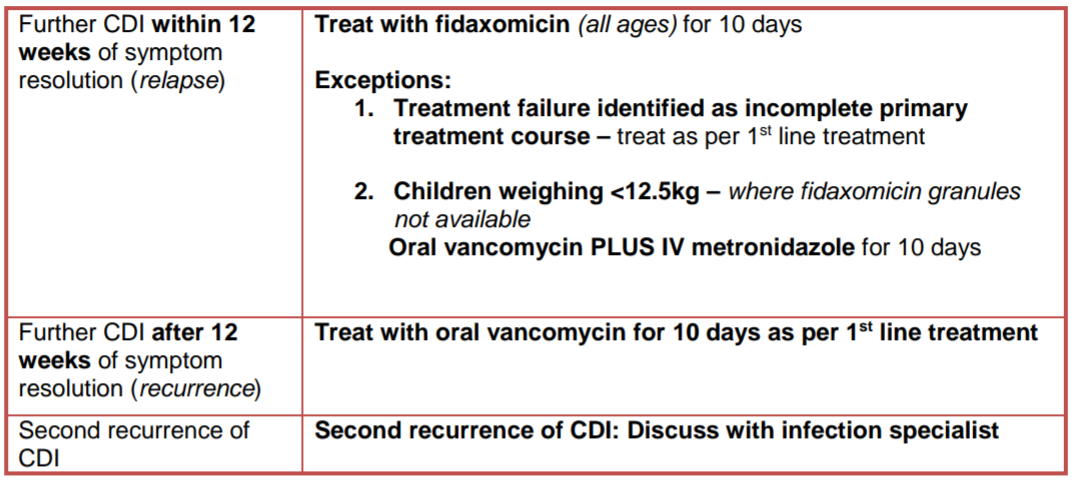
Management in Primary Care:
Metronidazole may be prescribed in community if delays in supply of oral vancomycin would result in delayed initiation of treatment. Metronidazole should be substituted with oral vancomycin as soon as availability is resolved to complete a total of 10 days treatment. For patients who cannot swallow vancomycin capsules, fidaxomicin should be considered the first line option in community setting.
Fidaxomicin:
Fidaxomicin tablets are licensed in children weighing at least 12.5kg. Fidaxomicin granules for oral suspension (40mg/ml) became available in the UK in May 2022. For children weighing less than 12.5kg consider oral vancomycin PLUS IV metronidazole if granules unavailable and/or seek specialist advice.
Vancomycin:
Vancomycin injection can be used via the oral route in the hospital setting where capsules are not available or where the calculated dose cannot be administered using available capsule strengths. Guidance on administration is available below:
To administer vancomycin via an NGT;
- Use vancomycin injection, 500mg vial.
- Reconstitute a 500mg vial with 10ml water for injection to give a 50mg/ml solution.
- Calculate the dose and withdraw the required amount from the vial. Discard the remainder of the vial.
- Flush the enteral feeding tube with 15mls sterile water prior to administration. The dose can be further diluted with water for injection if required.
- Administer the dose and flush with a further 15ml sterile water.