- Sputum or cough swabs are ideally obtained by CF Physiotherapists at CF clinic attendances, at Annual Review visits and on Day 1 of any IV antibiotic course.
- Additional cultures, using appropriate selective media may also be obtained whenever clinically indicated.
- Antibiotic sensitivities are reported and monitored
- Multi-resistant strains are sent to a reference laboratory approved by the Cystic Fibrosis Trust.
- Isolates of Pseudomonas aeruginosa, Burkholderia species and relevant NonTuberculous Mycobacteria are typed.
- Rates of acquisition of Pseudomonas aeruginosa, Burkholderia species and Multiresistant organisms in children attending the RHC Cystic Fibrosis clinic are monitored.
Microbiology Classification in Cystic Fibrosis Recommendations for Practice, Paediatrics (645)

Objectives
The principle of this document is to provide practical guidance for CF patient accommodation, appointments and movement throughout RHC which follows GGC IPC SOP advice.
Cystic fibrosis is a life-limiting disease, the outcome of which has improved significantly over the years. Progression of lung disease may be associated with certain respiratory bacteria, particularly Pseudomonas aeruginosa, Burkholderia species and Nontuberculous mycobacteria. Avoiding lung colonisation with these organisms is expected (although not proven) to improve life expectancy. The Cystic Fibrosis community has produced useful documents about these organisms. 2
STAPHYLOCOCCUS AUREUS
- Commonly found in throat and skin.
- May be transmitted via direct contact and via respiratory secretions, but not specifically from other people with Cystic Fibrosis.
- May cause acute respiratory exacerbations in Cystic Fibrosis.
- Oral prophylactic antibiotics are prescribed from diagnosis to prevent chronic colonisation.
HAEMOPHILUS INFLUENZAE
- May be found in the upper respiratory tract.
- May be transmitted via respiratory secretions, but not specifically from other people with Cystic Fibrosis.
- May cause acute respiratory exacerbations in Cystic Fibrosis.
- Intermittent additional second-line oral antibiotics are prescribed to eradicate this organism and to cover potential colonisation if patients develop cough.
PSEUDOMONAS AERUGINOSA
- May be found in the general environment, particularly around sinks and other moist areas e.g. soil and fresh water.
- May be transmitted between patients with Cystic Fibrosis through contact, e.g. with respiratory secretions.
- It is possible to be infected with more than one strain of Pseudomonas aeruginosa.
- May be associated with deterioration in respiratory function.
- May cause infection in other patient groups, e.g. those with burns and the immunocompromised.
- If present in wounds, should not pose a cross-infection risk if the wound is covered.
- If present in tracheostomy secretions, may pose a risk to CF patients, burns patients and immunocompromised patients.
- Eradication is attempted for any new culture after 6 months of clearance.
Conclusion:
- Patients with Pseudomonas aeruginosa pose a risk both to those children with Cystic Fibrosis who do not carry the organism and to children with Cystic Fibrosis who do.
- They also pose a risk to certain groups of children who do not have CF but have other conditions.
- Patients are considered positive for Pseudomonas aeruginosa until respiratory samples have been clear for at least 6 months or 12 months if eradication episodes have occurred more than twice.
BURKHOLDERIA SPECIES
- Are naturally found in soil around plant roots, rivers and lake sediments.
- Are transmitted between patients with Cystic Fibrosis ,mainly through close contact or contact with respiratory secretions
- May be associated with deterioration in respiratory function.
- It is possible to be infected with more than one Burkholderia species.
- Rarely causes disease in patient groups other than those with Cystic Fibrosis.
- If present anywhere in the Respiratory Tract in a patient who does not have CF, that patient must be nursed in Source Isolation if CF patients are or will be accommodated in that Ward.
Conclusion:
- Patients with B. species pose a risk both to those children with Cystic Fibrosis who do not carry the organism and to children with Cystic Fibrosis who do.
- Patients are considered positive for B. Species until sputum samples have been clear for at least 12 months.
NHS Greater Glasgow & Clyde Control of Infection Committee Standard Operating Procedure (SOP): IPC Precautions for patients with cystic fibrosis who have Pseudomonas spp and or B. cepacia
NON-TUBERCULOUS MYCOBACTERIA (NTM)
- These organisms are emerging as significant pathogens for people with CF.
- Mycobacterium abscessus in particular may be associated with worse clinical outcome.
- Patients are considered positive for NTM until at least 3 sputum samples taken at 2-3 monthly intervals have been clear within a 12 months period with macrolides being stopped for at least 2 weeks for screening samples.
- Patients who currently are considered positive for NTM in respiratory secretions are nursed in PPVL single rooms in a separate Ward from other CF patients and attend CF Clinics at separate times and in a separate clinical area from other CF patients.
NHS Greater Glasgow & Clyde Control of Infection Committee Standard Operating Procedure (SOP): IPC Precautions for patients with cystic fibrosis who have M. abscessus
MULTI-RESISTANT ORGANISMS
The following organisms are considered to be potentially multi-resistant in CF (MR).
CF patients will be considered colonised with these organisms until respiratory samples have been shown to be clear for 6 -12 months.
- MRSA
- Pandorea
- Achromobacter
- Stenotrophomonas maltophilia
- Other Pseudomonas species e.g. Ps fluorescens; Ps putida
- Any NTM e.g. M.abscessus
|
ORGANISM |
CLEAR IF -VE |
|
Pseudomonas aeruginosa |
6 months unless more than 2 eradication episodes in which case 12 months |
|
Burkholderia species |
12 months |
|
NTM- Any |
12 months with 3 –ve samples taken OFF Azithromycin |
|
Other Multiresistant CF Pathogens [MROther ] |
|
|
• MRSA |
12 MONTHS |
|
• Pandorea |
12 MONTHS |
|
• Achromobacter |
12 MONTHS |
|
• S. maltophilia |
12 MONTHS |
|
• Other Pseudomonas species |
6 MONTHS |
The GGC IPC Team has produced SOPs relating to CF patients attending RHC Inpatient and Outpatient departments. While adherence to the IPC advice in these SOPs allows for most CF patients to be seen in any Microbiology status order, exceptions to this are shown in the Table below which highlights the most significant pathogenic CF organisms.
- CF Patients in sets 1 and 2 should wherever possible be seen in order 1-2.
- CF patients in sets 3-5 must be seen in the order 3-5 in all circumstances.
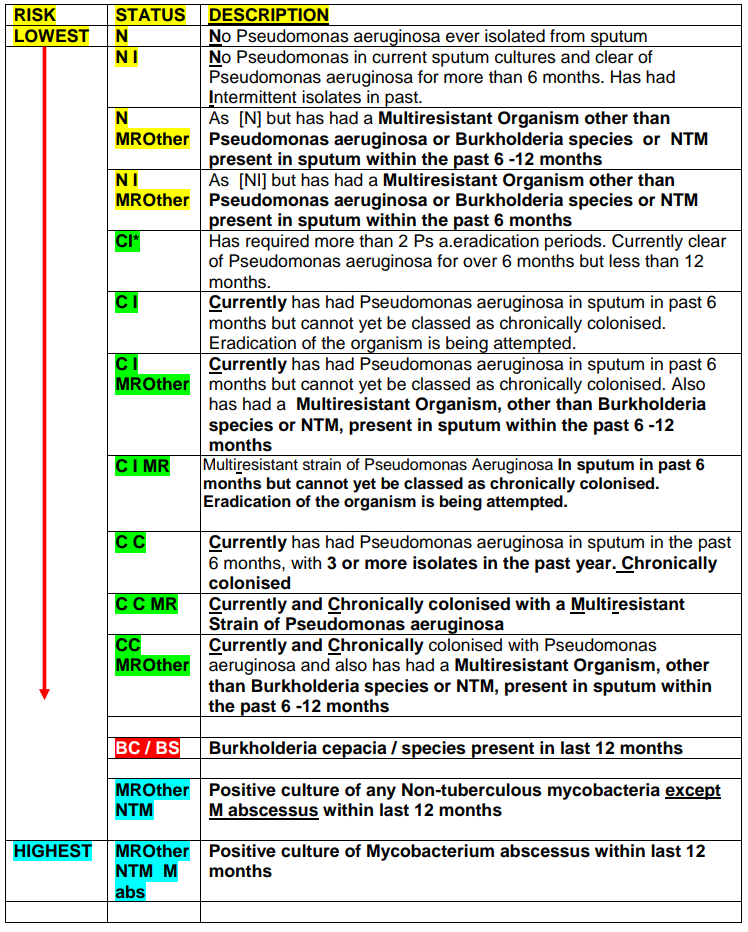
- All other CF patients should ideally be seen in an order of Low to High microbiological risk as per CF Microbiology Status list – see page 10.
- CF Patients should not be cohorted.
|
Order |
|
Microbiology Status |
|
1 |
Multiresistant CF pathogens* ( see above) apart from Pseudomonas, Burkholderia, or any NTM – Microbiology status [MROther] |
|
|
2 |
Pseudomonas aeruginosa- Microbiology status [CI/CI*/CC] |
|
|
3 |
Burkholderia Species – Microbiology status [BC / BS ] |
|
|
4 |
Any Non-tuberculous Mycobacteria except M abscessus Microbiology status [ MROther NTM] |
|
|
5 |
M abscessus – Microbiology status [MROther NTM M abscessus] |
CLINICS
- Appointment times must be kept to reduce risk of cross-infection. If patients arrive more than 15 minutes out -with their appointment time, they may not be seen at that Clinic.
- The RHC CF Clinic appointment template is based on average CF Clinic patient journey times which have been audited by the CF team.
- Changes or cancellations to appointments must be made via the CF Unit Administrator Tel: 0141 451 6546
- Patients coming to RHC Cystic Fibrosis Clinics should enter the Hospital via the Main Entrance.
- On arrival at RHC, patients should go directly to Area 1 where they will be shown to a Clinic room.
- Patients are asked not to use Self Check-In desks or visit the Atrium area or other communal Hospital areas e.g. Shops, Restaurants etc.
- Staff will adhere to GGC IPC guidance regarding hand hygiene and cleaning of consulting rooms and equipment.
NON-CF CLINIC APPOINTMENTS / OTHER VISITS TO RHC
- In order to minimise cross-infection risk, parents are asked to notify the CF Nurse Specialists / CF Unit Administrator of any non-CF Clinic appointments their child has to attend at RHC.
- Patients with CF should not be brought to RHC for visits other than their own appointments.
Cystic Fibrosis patients are admitted to one of 4 Designated Wards in RHC- 3A,3B,3C,2C(ARU) - as per Microbiology Status displayed as an Alert on Trakcare. If there is a clinical requirement for a CF patient to be nursed in another Ward e.g. Renal, Surgical, Cardiac, this will be managed on an individual basis and CF IPC SOP policies adhered to.
- All CF patients are nursed in single rooms with en-suite facilities.
- Patients admitted to Ward 3C are nursed in PPVL rooms.
| WARD 3B: (N / NI / NMROther / NIMROther ) |
| WARD 3A: (CI /CI*/ CI MR/ CI*MR/ CC / CCMR / CI MROther/ CI*MROther/ CCMROther ) |
| WARD 2C (ARU): (BC / BS) |
| WARD 3C: ANY NTM |
ENSURING A UNIFORM STANDARD OF CARE IN EACH WARD
A Cystic Fibrosis Care Plan is available for Staff to follow. This is available in hard copy format on each Ward and is maintained by the CF Sisters. When possible, the Cystic Fibrosis Nurse Specialists ensure that there are regular teaching sessions about Cystic Fibrosis for the nurses on the each of the different wards.
ACCESS FOR VISITORS/CARERS
- Siblings, who have CF and who currently attend RHC CF Unit may be allowed to visit after discussion with the CF Nurse Specialists.
- Visitors who have Cystic Fibrosis themselves should not visit any patient with CF within RHC.
- Visitors should not have contact with other patients with Cystic Fibrosis within the hospital.
GROUP ACTIVITIES WITHIN RHC
For infection control reasons patients with CF should not be taken to Hospital Group Activities or communal patient areas such as Medicinema, Play Areas (including within the RHC Atrium), School Room, Radio Lollipop, Shops, Coffee Bars or Restaurants, etc.
Arrangements must be in place to ensure that CF patients do not mix in waiting areas of other departments. Ward Staff should contact individual Departments to check that no other CF patients are currently attending that Department.
Arrangements will be made to ensure that CF patients do not mix in the admission or recovery areas of theatres. Patients with multi-resistant organisms should be placed at the end of the appropriate theatre list.
All staff should familiarise themselves with this policy and relevant GGC IPC SOPs.
OUTPATIENTS
- RHC CF Outpatient Clinics are usually on Monday and Thursday mornings starting at 9am in Area 1.
- Appointment times are allocated to minimise cross-infection risk. If patients are late for their appointment, this may need to be rescheduled for another day ( See CF Clinic Template agreed with RHC ICP Team March 2016)
- On arrival at RHC, patients are asked to go directly to Area 1 where they will be shown to a Clinic room.
- All Clinic procedures will be carried out in patients’ own clinic rooms.
- Patients are asked not to use Self Check-In desks or visit the Atrium area or other communal Hospital areas e.g. Shops, Restaurants etc.
- Regular monitoring of cough swabs/sputum cultures is undertaken at clinics.
- If parents require to change CF clinic appointments or if their child has any non-CF Clinic appointments at RHC they are asked to contact the CF Unit administrator (Tel 0141 451 6546 ) rather than the appointments office at RHC
- To minimise cross-infection risk, RHC CF Patients are asked not visit the Queen Elizabeth University Hospital unless specifically arranged by RHC CF Team.
INPATIENTS
- Patients with CF are admitted to one of four designated wards in RHC depending on Microbiology status. These Wards are 3A, 3B, 3C on the Third Floor and the Acute Receiving Unit (2C) on the Second Floor.
- Staff can check on Trakcare Alerts to find the Microbiology Status and Designated Ward allocated to each CF patient. This is updated regularly.
- Patients are accommodated in single rooms with en-suite facilities. To minimise cross-infection, patients are asked to stay in their rooms where they will receive all their care. They will not have access to other ward areas or communal patient areas within the hospital during their stay unless supervised by CF Team members or when attending other hospital Departments for investigations as per NHSGGC IPC guidance.
- In keeping with NHSGGC infection control guidance, patient room doors must be kept closed at all times.
- For infection control reasons patients with CF should not be taken to Hospital Group Activities or communal patient areas such as Medicinema, Play Areas (including within the RHC Atrium), School Room, Radio Lollipop, Shops, Coffee Bars or Restaurants, etc. unless specifically arranged by GGC IPC Team.
- For infection control reasons patients with CF attending RHC are also advised not to visit Queen Elizabeth University Hospital unless specifically arranged by RHC CF Team.
- Visitors (including siblings) who have Cystic Fibrosis and who do not attend RHC CF Unit should not visit any patient with CF within RHC.
- Patients’ siblings who have CF and who currently attend RHC CF Unit may be allowed to visit after discussion with the CF Nurse Specialists.
- Visitors, including parents of CF patients, should not have contact with other patients with Cystic Fibrosis within RHC.
OUTPATIENTS
- RHC CF Outpatient Clinics are usually held on Monday and Thursday mornings starting at 9am in Area 1 which is situated on the left of the RHC Main Entrance.
- Appointment times are strictly allocated to minimise cross-infection risk. If patients are late for their appointment , this may need to be rescheduled for another day .
- On arrival at RHC, patients should go directly to Area 1 where they will be shown to a Clinic room.
- All Clinic procedures will be carried out in patients’ own clinic rooms.
- Patients are asked not to use Self Check-In desks or visit the Atrium area or other communal Hospital areas e.g. Shops, Restaurants etc.
- Regular monitoring of cough swabs/sputum cultures is undertaken at clinics.
- If parents require to change CF clinic appointments they are asked to contact the CF Unit administrator (Tel 0141 451 6546 ) rather than the appointments office at RHC
- Patients with CF should not be brought to RHC for visits other than their own appointments. Please let the CF Sisters know if attending non-CF clinic appointments.
- To minimise cross-infection risk, RHC CF Patients are asked not visit the Queen Elizabeth University Hospital unless specifically arranged by RHC CF Team.
INPATIENTS
- Patients with CF are admitted to one of four designated wards in RHC depending on Microbiology status. These Wards are 3A, 3B, 3C on the Third Floor and the Acute Receiving Unit (2C) on the Second Floor.
- Staff will check on Trakcare Alerts to find the Microbiology Status and Designated Ward allocated to each CF patient. This is updated regularly.
- Patients are accommodated in single rooms with ensuite facilities. To minimise cross-infection, patients are asked to stay in their rooms where they will receive all their care.
- In keeping with NHSGGC infection control guidance, patient room doors must be kept closed at all times.
- For infection control reasons patients with CF should not be taken to Hospital Group Activities or communal patient areas such as Medicinema, Play Areas (including within the RHC Atrium), School Room, Radio Lollipop, Shops, Coffee Bars or Restaurants, etc. unless specifically arranged by CF Team / GGC Infection Control Team members or when attending other hospital Departments for investigations..
- For infection control reasons patients with CF attending RHC are also advised not to visit Queen Elizabeth University Hospital unless specifically arranged by RHC CF Team.
- Visitors (including siblings) who have Cystic Fibrosis and who do not attend RHC CF Unit should not visit any patient with CF within RHC.
- Patients’ siblings who have CF and who currently attend RHC CF Unit may be allowed to visit after discussion with the CF Nurse Specialists.
- Visitors , including parents of patients who have CF, should not have contact with other patients with Cystic Fibrosis within hospital.