Preterm infants commonly develop respiratory distress syndrome (RDS) requiring some form of respiratory support and potentially surfactant administration1. Treatment with surfactant has been shown to reduce the risk of death and bronchopulmonary dysplasia (BPD) in preterm infants; however the standard approach to administering surfactant involves using an endo-tracheal tube and a period of mechanical ventilation (MV)2. It is well known that MV causes damage to the fragile preterm lungs so many lung protective strategies for respiratory management and ventilation have been developed.
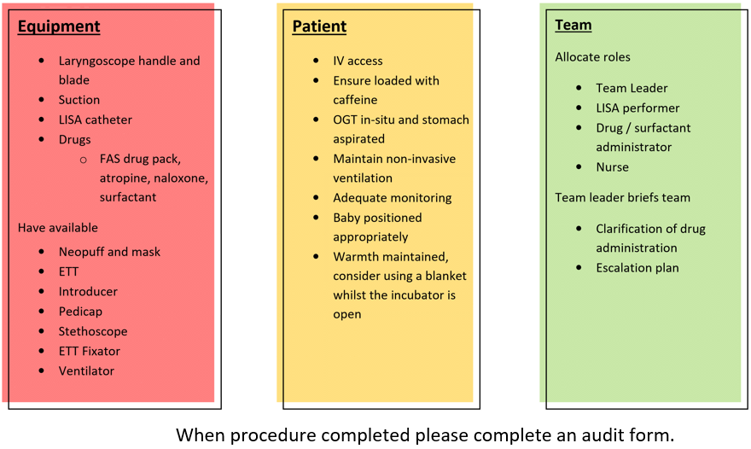
As per our current guideline on the respiratory management of preterm infants the preferred initial management is using primary CPAP with the use of rescue surfactant for infants with an increasing oxygen requirement. To reduce the need for MV but still deliver surfactant several less invasive techniques have been developed. One of these techniques is called LISA (Less invasive surfactant administration) and is the process of delivering surfactant directly into the lungs via a fine bore catheter inserted into the trachea3. In a recent review by Wu et al4, LISA technique was associated with a significant reduction in the risk of mechanical ventilation within 72hours and bronchopulmonary dysplasia at 36 weeks compared to other strategies of surfactant administration (p<0.00001). These findings have been also been seen in a meta-analysis by Isayama et al in 20151 and most recently by Aldana-Aguirre et al who found a lesser need for MV and a reduction in the composite outcome of death or BPD at 36 weeks compared to INSURE (p 0.01)2.