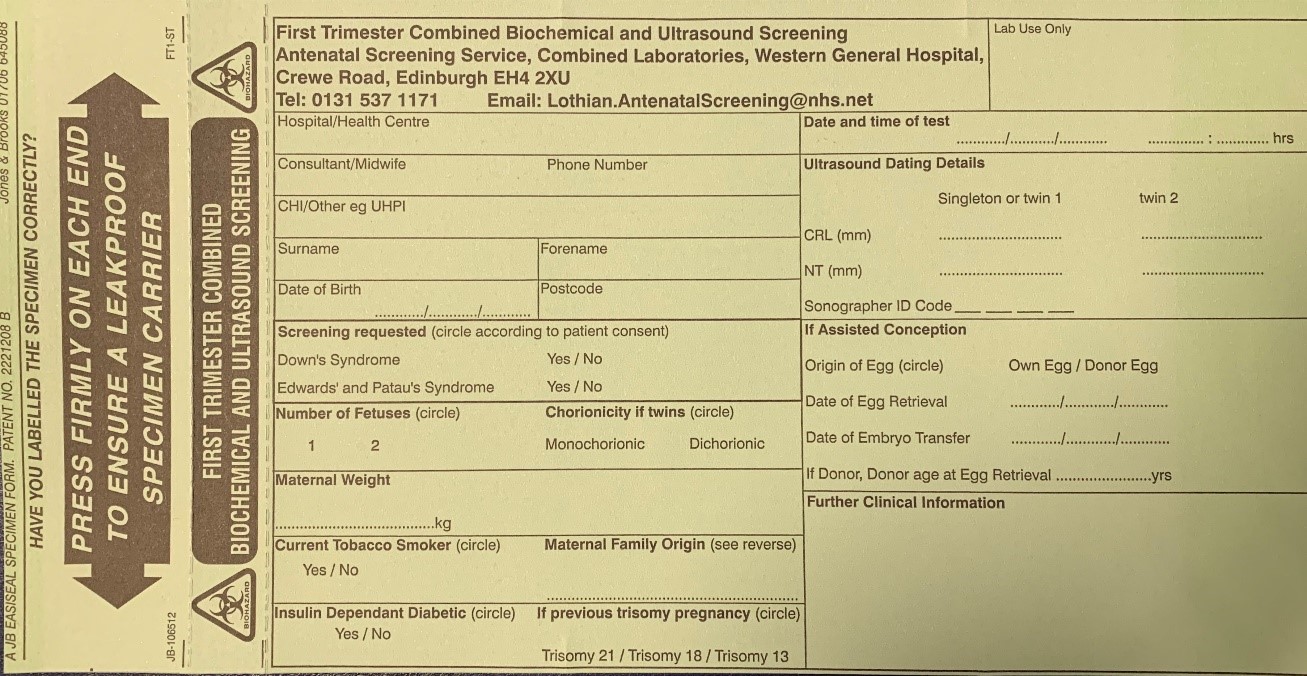
Where both dating and 1st trimester screening are requested and the CRL is between 45.0 and 84.0mm, the pregnancy should be dated using the CRL measurement.
Criteria for measurement of the fetal crown rump length (CRL) as part of the combined 1st trimester screening programme
The CRL range should be between 45.0 and 84.0 mm.
The magnification of the fetus should be as large as possible clearly demonstrating the entire crown-rump length.
A midline sagittal section of the whole fetus should be obtained with the fetus horizontal on the screen, either supine or prone. The fetus should be in a neutral position with fluid visible between the fetal chin and chest, neither hyper extended nor flexed.
The best of three measurements should be taken. Linear callipers should be used to measure the maximum un-flexed length. Intersection of the callipers (+) should be placed on the outer margin of the skin borders of the CRL. Two images of the measured CRL must be retained, one for the patient record and one for audit purposes.
If the CRL is < 45.0mm re-appoint the patient within the 11+2 – 14+1 weeks screening window.
If the CRL is > 84.0mm arrange appointment for 2nd trimester biochemistry screening and date pregnancy using Head Circumference (HC).
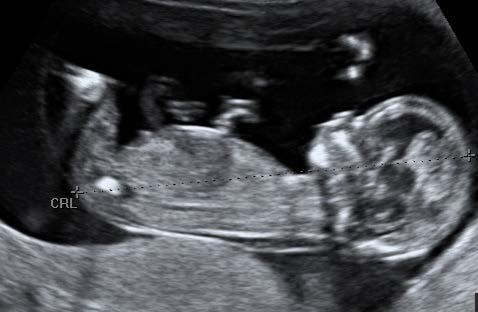
The NT Measurement
The NT measurement should only be performed if a CRL measurement, which meets the recommended NHS FASP criteria for CRL has been obtained.
Criteria for measurement of the fetal nuchal translucency (NT) measurement
A midline sagittal section of the fetus should be obtained. The fetus should be horizontal on the screen, either supine or prone.
Care must be taken to distinguish between fetal skin and amnion. The fetus should be in a neutral position.
The image should be magnified, such that only the fetal head and upper thorax occupy the whole screen. In magnifying the image (pre- or post-zoom) it is important to turn down the gain.
The widest part of the translucency must always be measured. Measurements should be taken with the horizontal lines of the callipers placed ON the lines that define the NT thickness.
During the scan more than one measurement must be taken and the maximum one which meets all the criteria should be recorded. Two images of this measured NT should be retained, one for the patient record, one for audit purposes.
If the NT measurement is ≥ 3.5mm, perform combined screening test and follow pathway for raised nuchal translucency (NT) ≥ 3.5mm
Too early/late/unable to obtain measurements
Too Early: CRL measurement <45.0mm – re-appoint at appropriate gestation.
Too late: CRL measurement >84.0mm – arrange appointment for 2nd trimester biochemistry (≥ 15+0 weeks) screening and date pregnancy using Head Circumference (HC).
Unable to obtain measurements – offer a 2nd attempt. This second attempt at screening should be on the same day.
If unable to obtain measurements after two attempts, explain limitations of scan and record on report. Arrange 2nd trimester dating scan to coincide with biochemistry appointment.
Ultrasound Images
One set of paired CRL and NT images to be inserted into brown image envelope in patient notes, one set to be kept aside for audit purposes.
Ultrasound Report
The Ultrasound report should be documented on Badgernet under ‘Key Notes – New Ultrasound Note’. Ensure all appropriate fields are filled including the authorization box (your digital signature) for the report to be valid.
If unable to obtain NT measurements indicate reason for failed attempt i.e. poor views due to fetal position. If patient to be re-appointed for 2nd trimester screening enter suggested date.
Medical Genetics Form (Appendix A)
Attach a patient label, with name, address, DOB and CHI number to the Medical Genetics First Trimester Combined Ultrasound and Biochemical (CUB) Screening form.
Enter the following data:
- Hospital
- Consultant
- Maternal Weight
- Number of Fetuses
- Chorionicity if multiple pregnancy
- Maternal Family Origin
Indicate YES or NO for the following categories:
- Screening required – Down’s Syndrome T21
- Screening required – Edwards’ Syndrome T18 and Patau’s Syndrome T13
- Current Smoker
- Previous Trisomy Pregnancy
- IDDM
Complete the Ultrasound Details section;
- Date of scan
- Estimated date of delivery
- CRL (mm)
- NT (mm)
- Ultrasonographer code
If assisted conception pregnancy, record all relevant details in the Assisted Conception section
“Date of sample” and “Sample taken by” fields to be entered by the Midwife/HCSW who performs the venipuncture.
Medical Genetics form to be passed to the Midwife/HCSW for completion and sent together with the biochemistry sample to Medical Genetics Labs.