A | AIRWAY | (Jaw thrust, High flow O2) & LATERAL TILT & CALL FOR HELP 2222 |
B | BREATHING | Assess, ventilate as required. |
C | CIRCULATION | Assess (BP, pulse, O2 saturation) & Access
C1: CONTROL SEIZURE C2: CONTROL BLOOD PRESSURE C3: CONTROL FLUID BALANCE *** The “Eclampsia” box contains all the necessary drugs, antidotes and instructions *** |
D | DELIVERY PLAN | |
E | EVALUATE | Reassess monitoring and Investigations |
Hypertension Eclampsia Management (402)

Please report any inaccuracies or issues with this guideline using our online form
Eclampsia is a life threatening complication of pregnancy.
UK incidence is about 1 per 2000 maternities.
Seizure incidence: 38% pre-labour, 18% during labour, 44% postnatal
Pathophysiology is thought to involve cerebral vasospasm leading to ischaemia, disruption of the blood brain barrier and cerebral oedema.
Neurological complications may include coma, focal motor defects and cortical blindness. Cerebrovascular haemorrhage is a complicating factor in 1- 2 % of cases.
Eclampsia is usually part of a multisystem disorder. There may be little or no warning that a seizure is imminent and no evidence of existing "pre-eclampsia". All pregnant women regardless of parity are at risk. There is a significant increased risk in women in the 15-19 year age group and women with previous eclampsia.
Magnesium Sulphate is the drug of choice unless there are specific contra indications to its use.
Magnesium Sulphate:
Loading Dose (by hand):
- 4 grams IV over 5 minutes
(Add 4 grams (8 mls of 50%) Magnesium Sulphate to 12 mls Normal Saline)
Maintenance Infusion Dose:
- IV infusion 1 gram Magnesium Sulphate per hour
Maintenance Infusion Preparation:
- 10 grams (20 mls of 50%) Magnesium Sulphate made up to 50 mls by adding to 30 mls normal saline in a 60 ml luer lock syringe
- Infusion rate is 1 gram (5 mls) per hour via an syringe driver
Infusion is maintained at 1 gram/hr for 24 hours provided:
- Respiratory rate > 14 per minute
- Urine output > 25mls/hour, and
- Patellar reflexes are present
NB: The volume of the Magnesium Sulphate infusion must be included as part of the total fluid maintenance infusion for the patient of 85ml/hour
Recurrent Seizures on Treatment:
- Give a 2nd bolus dose of Magnesium Sulphate 2 grams over 5 minutes by hand (do not stop infusion)
- add 2 grams (4 mls of 50%) Magnesium Sulphate to 6 mls of Normal Saline
- One dose only
If further seizures despite 2nd bolus give Diazepam 10mg IV. Intubation may be required to protect airway and ensure adequate oxygenation.
Magnesium Sulphate – Patient Monitoring:
Reflexes:
- Patellar reflexes after completion of loading dose and hourly whilst on maintenance dose (use arm reflexes if functional regional anaesthesia).
- If reflexes are absent stop infusion until reflexes return and check Magnesium level.
Oxygen Saturation / Respiratory Rate:
- Continuous O2 saturation should be assessed.
- Perform respiratory rate every 15 minutes
- If O2 saturation < 94% or respiratory rate < 14 / min, administer O2 (4 L/min via Hudson mask), stop Magnesium Sulphate infusion and call anaesthetist. Check Magnesium level. Consider antidote
Urine Output:
Monitor hourly.
If >20 ml/h - continue Magnesium Sulphate infusion.
If 10 - 20 ml/h & creatinine <150mmol/l - continue as protocol and recheck Magnesium level every 2 hours.
If 10 - 20 ml/h & creatinine > 150mmol/l (or urea >10) - recheck Magnesium levels immediately and every 2 hours. Decrease infusion rate to 0.5gram/hour.
If < 10 ml/h - stop infusion and check Magnesium level.
Biochemical Monitoring (Magnesium levels): This is not routine. If required then see below.
The Therapeutic range is 2-4 mmol/l.
Low If < 2 mmol/l - Maintain infusion at current rate. Recheck in 2 hours.
Therapeutic If 2 -3.5 mmol/l - Continue infusion at current rate. Recheck in 2 hours if clinical indication remains.
High If 3.55 - 5 mmol/l - STOP INFUSION for 15 min and then recommence at half previous infusion rate and recheck in 1 hour.
Very High If > 5mmol/l - STOP INFUSION and consider antidote. See below for further details.
Magnesium Sulphate toxicity and management:
Clinical Features | Mg level | Action |
Loss of Patellar reflexes | circa 5 mmol/l | STOP INFUSION GIVE ANTIDOTE |
Muscle Paralysis | circa 6-7.5 mmol/l | STOP INFUSION GIVE ANTIDOTEAS ABOVECHECK |
Respiratory Arrest | circa 12 mmol/l | STOP INFUSION INSTITUTE CPR |
- MAP > 140 mm Hg is an obstetric emergency
- No evidence that one particular drug is superior for treatment. Labetalol tends to be the first line drug of choice in this locality.
- Continuous fetal monitoring is necessary because lowering of maternal BP may lead to fetal distress, particularly if there is associated IUGR
- Automated oscillometric devices may underestimate BP
- Commence MEWS chart (one large bold box per hour)
- MAP >140 mm Hg - measure BP every 5 minutes
- MAP 125 -140 mm Hg - measure BP every 15 minutes
- Aim for gradual reduction in BP to around 130-140 / 90 - 100 mmHg (MAP < 125)
- Site 2 x wide bore IV cannula & check BP bloods (U+E, LFT, urate, FBC +/- coagulation if platelet count is < 150 or previous abnormality) 6 hourly if patient stable, X-match 2 units blood
- Foley catheter inserted and hourly urine volumes commenced
- Continuous pulse oximetry
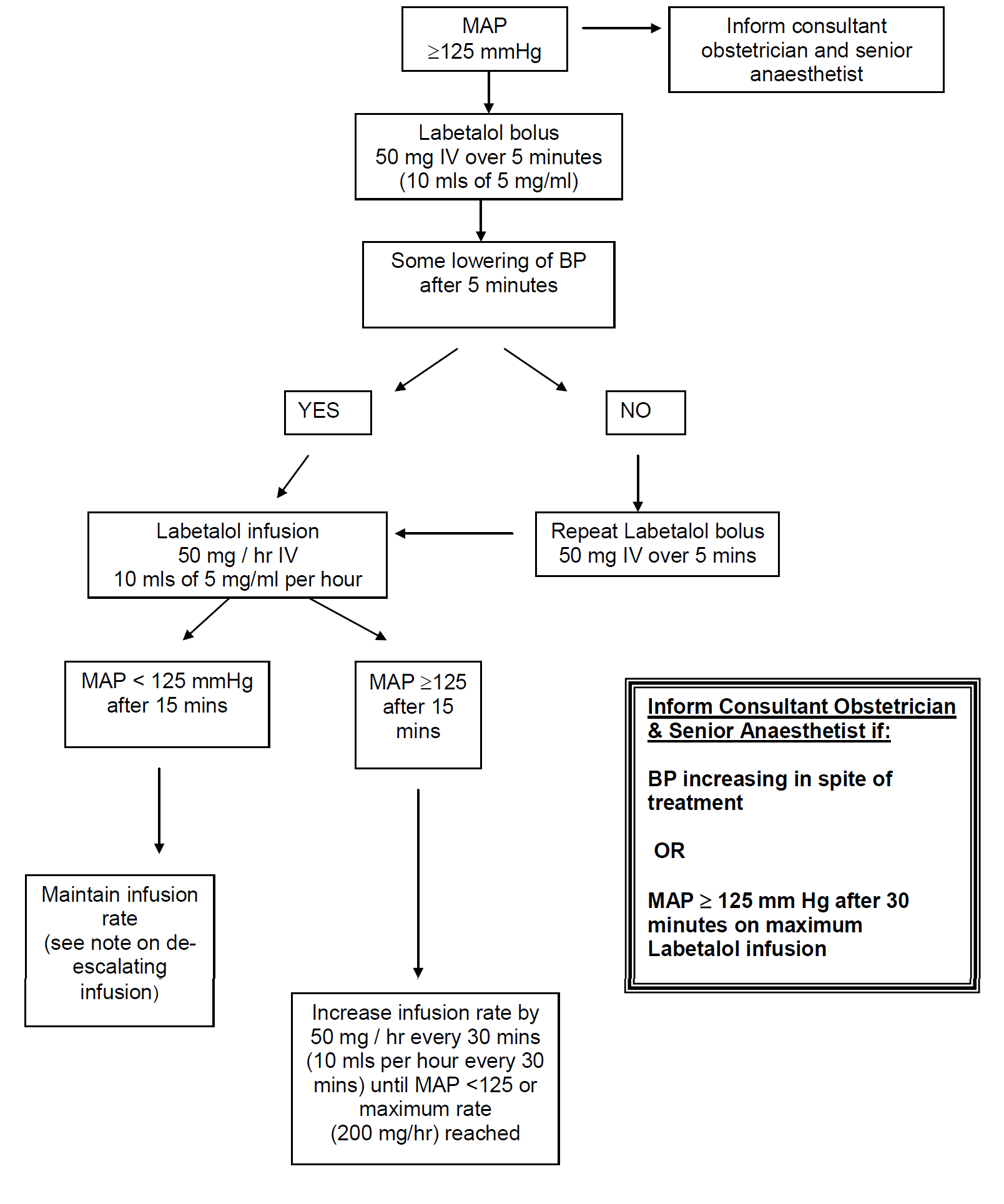
Labetalol:
|
Contraindications including: |
Asthma, Bronchospasm, Uncontrolled heart failure |
|
IV bolus: |
50 mg over 5 minutes |
|
Infusion preparation: |
Prepare 5 mg/ml infusion |
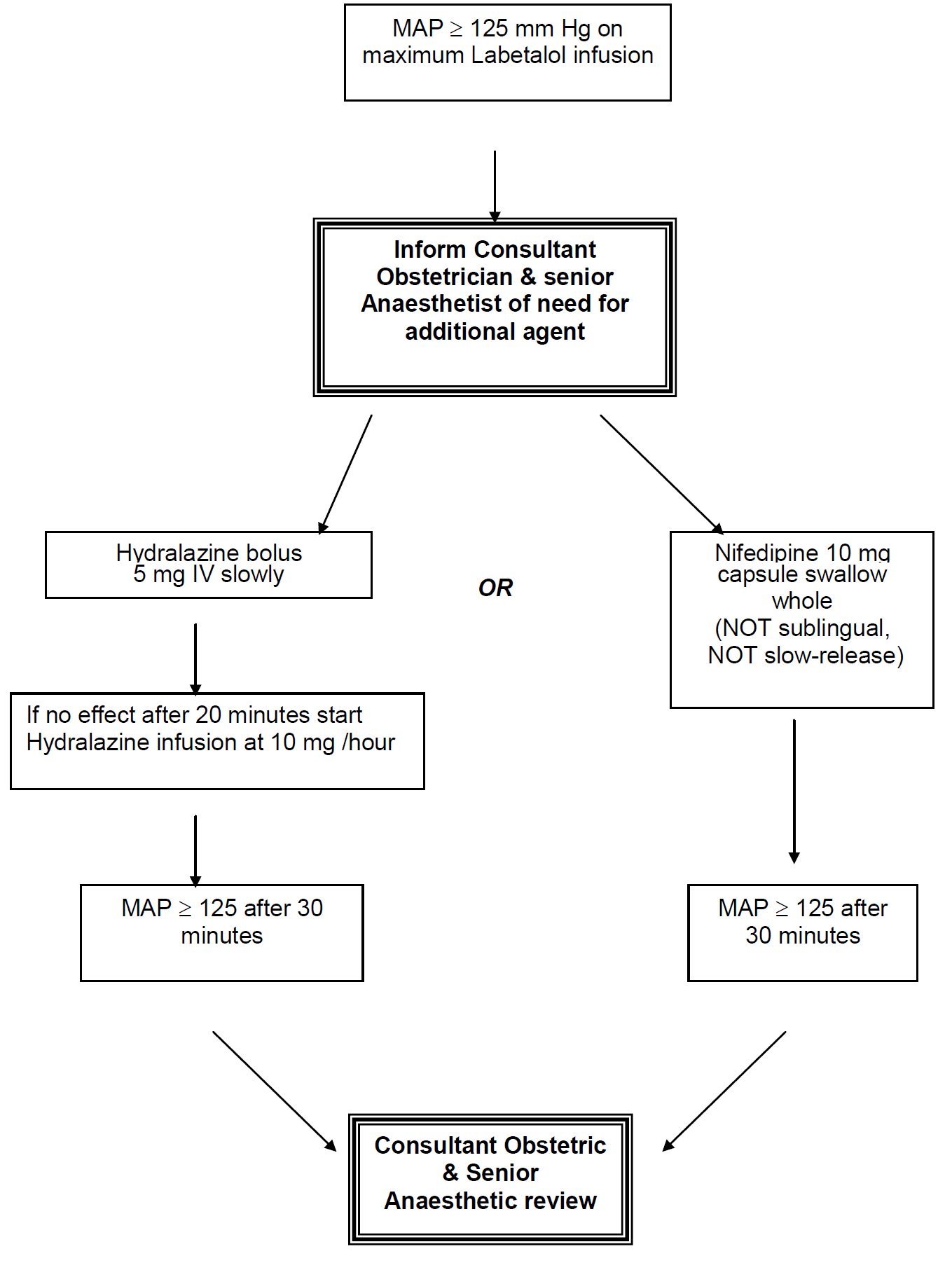
Nifedipine:
| Contraindications including: | Hypersensitivity to nifedipine, or to other dihydropyridines because of the theoretical risk of cross-reactivity, or to any of the excipients, Angina, Recent MI, Aortic Stenosis |
| (Care with Magnesium Sulphate – see note below*) | |
| Preparation: | 10 mg capsule orally (swallowed whole)Repeated doses of 10 mg can be given at 6 hourly intervals |
Hydralazine:
| Contraindications: | Hypersensitivity to hydralazine or dihydralazine Connective tissue disorders Severe tachycardia and heart failure with high output cardiac failure (e.g. in thyrotoxicosis) Myocardial insufficiency due to mechanical obstruction (e.g. in the presence of aortic or mitral stenosis or constructive pericarditis) Isolated right ventricular failure due to pulmonary hypertension (cor pulmonale) Dissecting aortic aneurysm |
| IV bolus: | 5 mg slowly over 5 minutes Boluses can be repeated at 20 minute intervals, but may be simpler to start infusion( A 5 mg dose can be effective for 6 hours) |
| Infusion preparation: | Prepare 1 mg/ml infusion i.e. 40 mg Hydralazine made to 40mls with normal saline. Infuse at 10 mg (10 mls) per hour |
*Note
There are 2 case reports of neuromuscular blockade resulting from simultaneous use of Nifedipine and Magnesium Sulphate. However, 1,469 women were assigned to receive Magnesium Sulphate and Nifedipine in the Magpie trial, and no such blockade was reported. Similarly, no adverse events were reported in RCTs comparing Hydralazine with Nifedipine in which all, or some, women received magnesium sulphate. The risk of neuromuscular blockade is therefore likely to below. MAP > 140 mm Hg is an obstetric emergency.
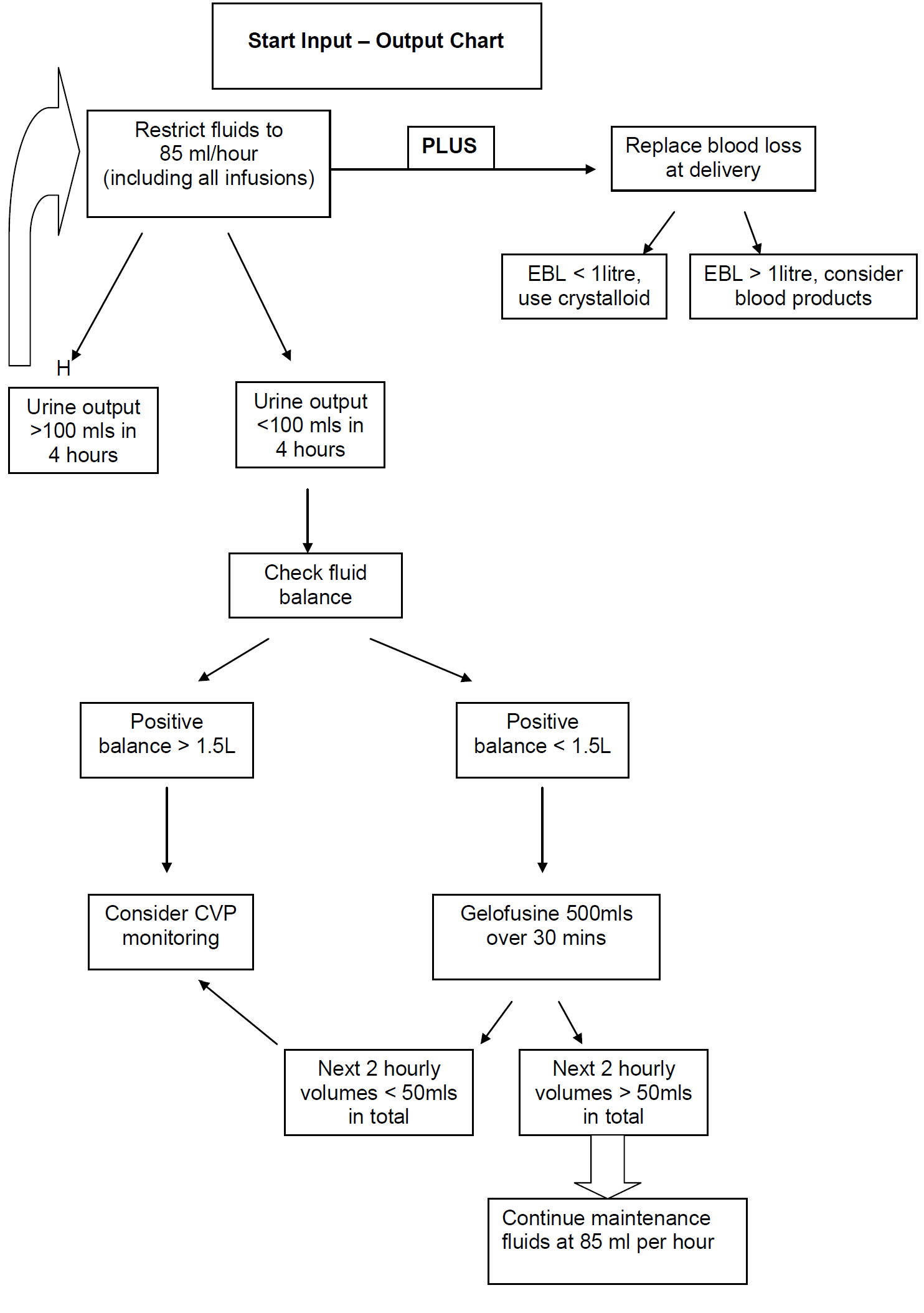
- The main risk is of pulmonary oedema to iatrogenic fluid overload.
- Patients should be fluid restricted (85mls per hour of total input).
- Document hourly urine output on MEWS chart
- Oliguria is common in severe pre-eclampsia.
- The natural diuresis may not occur for at least 12 hours post delivery.
- Renal failure is uncommon.
- Furosemide should be reserved for pulmonary oedema and prescription must be discussed with consultant obstetrician.
- In persisting oliguria U&Es should be checked 6 hourly.
- In persisting oliguria: urine osmolality that is not concentrated, or high potassium levels indicates renal failure and renal physicians should be contacted.
- CVP monitoring can be misleading.
- Consultant obstetrician on-call must be informed if CVP line is considered.
- Delivery is the definitive treatment for severe pre-eclampsia/eclampsia
- Mother MUST be stabilised prior to delivery irrespective of circumstances (e.g. fetal distress)
- HDU support is required post delivery
- ITU if ventilated
N.B. Avoid ergometrine use. Skip to next medical treatment if uterine atony (PPH guideline).