History
A full medical history is required including smear history. Particular attention should be paid risk factors for endometrial hyperplasia or cancer, medication history including type and compliance with hormone replacement therapy (HRT) regime, current or past tamoxifen use and use of anticoagulants such as warfarin, heparin, clopidogrel or DOAC therapy.
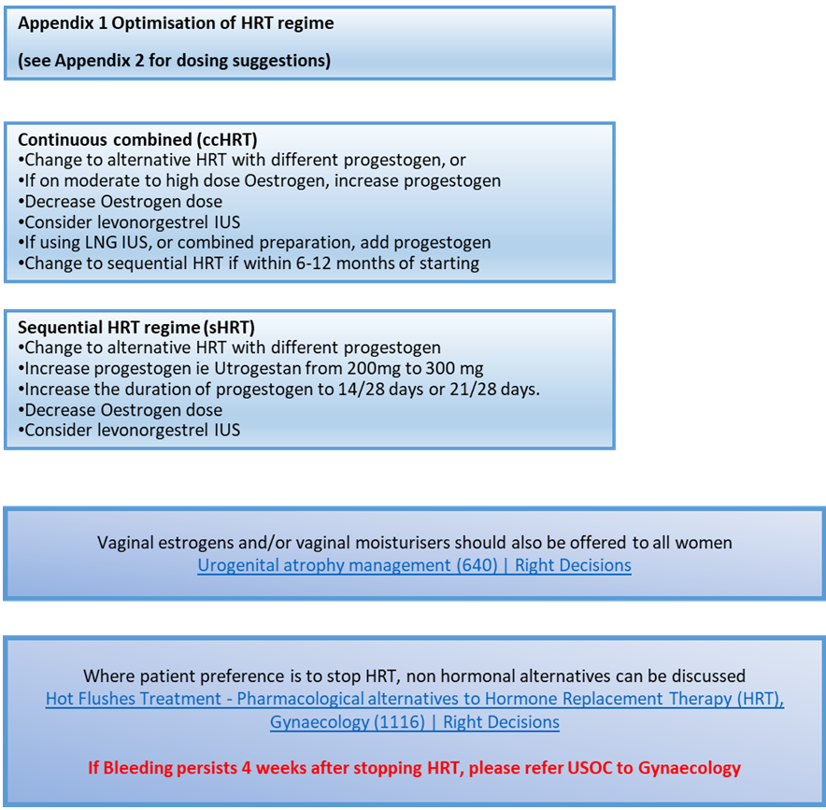
Optimisation of modifiable factors can, in themselves, reduce episodes of unscheduled bleeding on HRT and endometrial cancer risk.
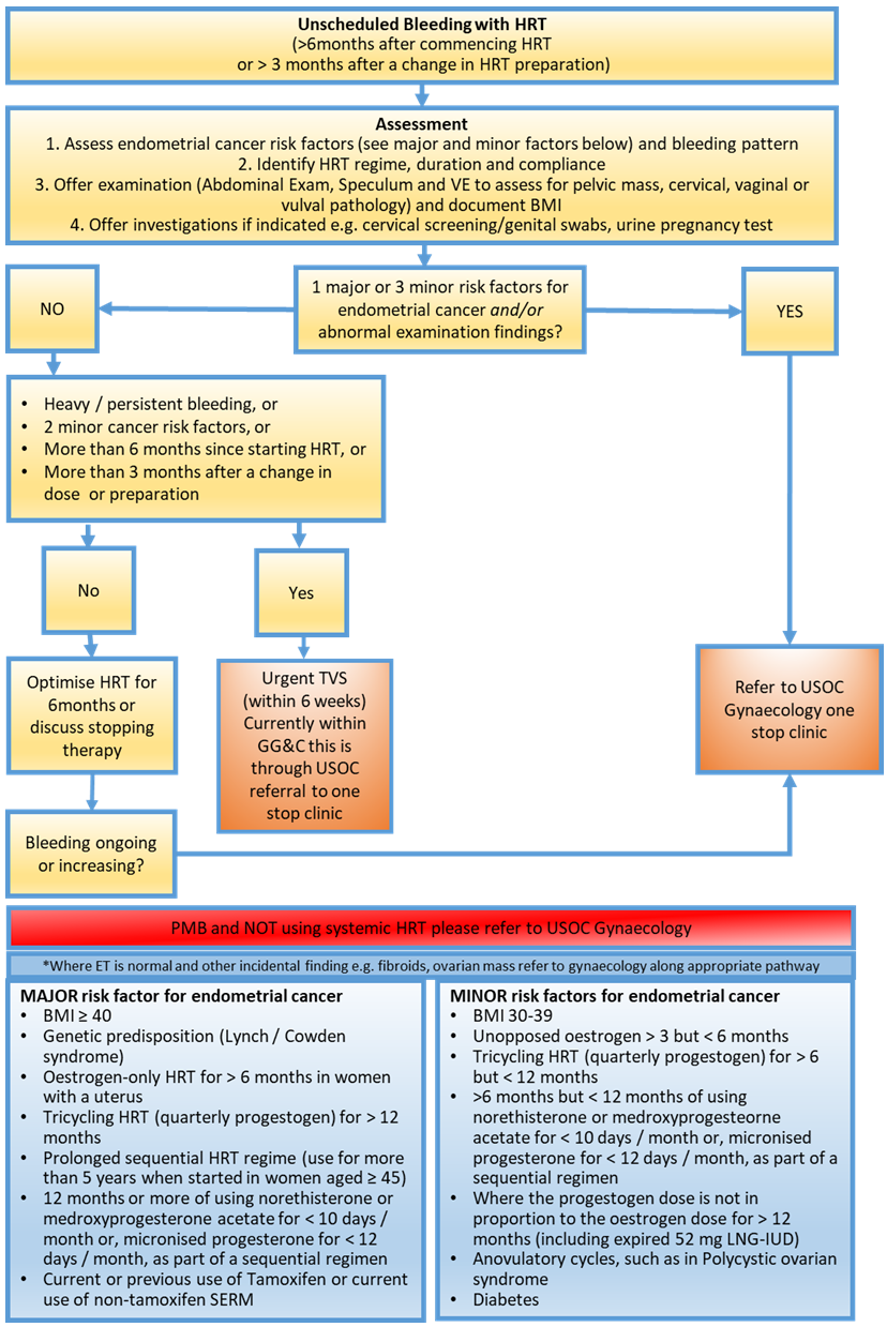
The British Menopause Society consider risks for endometrial pathology as Major and Minor outlined in the table in Figure 1.
Examination
All women with PMB or unscheduled bleeding with HRT should have their measure of Body Mass Index (BMI) updated as this helps determine risk stratification.
Pregnancy testing should be considered in those <55 years of age Pregnancy Testing in Gynaecology patients (316) | Right Decisions
Women should have an assessment of their entire genital tract including vulva, vagina and cervix to assess for localised pathology. To facilitate examination appropriately sized speculum and provision of sufficient vaginal lubrication/use of vaginal moisturisers should be considered.
A bimanual pelvic examination should also be undertaken to assess for the presence of any pelvic mass
A cervical smear should only be taken if it is due according to screening recall dates.
In secondary care, patients may need to have an examination under anaesthetic if despite conservative supportive measures, if examination proves impossible in the outpatient setting.
Investigations
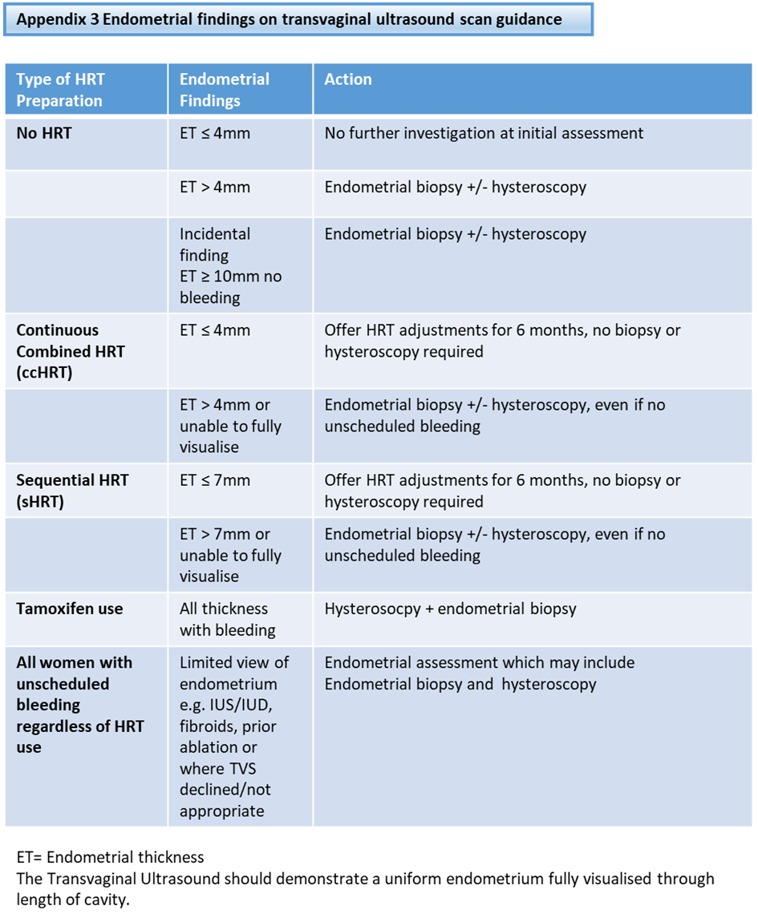
Guidance for indications for each investigation is outlined in Appendix 3.
Imaging
The initial investigation of choice is the measurement of endometrial thickness (ET) by transvaginal ultrasound scan (TVUSS) in the long or sagittal plane.
Transabdominal ultrasound scan may be used as a complementary investigation e.g. if the uterus is particularly enlarged or a wider view of the abdomen is required. It may also be used in the assessment of the small number of women in whom transvaginal scanning is technically impossible, however, TAUSS is not considered gold standard for assessment of endometrial thickness and direct assessment of endometrium by hysteroscopy and biopsy should be considered.
Where there are limited view of endometrium (e.g. IUS/IUD, fibroids, prior ablation) or where TVS declined/not appropriate (e.g. transgender men, or previous sexual assault), referral should be considered for endometrial assessment by endometrial biopsy +/- hysteroscopy.
Tamoxifen and TVUSS
Tamoxifen can be associated with distortion of the endometrial architecture. Consequently, ultrasonography is a poor means of detecting endometrial cancer in this group. All such women should undergo hysteroscopy and endometrial biopsy.
Where other non tamoxifen Selective Estrogen Receptor Modifiers (SERMs) medications are used, women should be investigated in the same way as those taking tamoxifen i.e. undergo hysteroscopy and endometrial biopsy.
Endometrial Biopsy
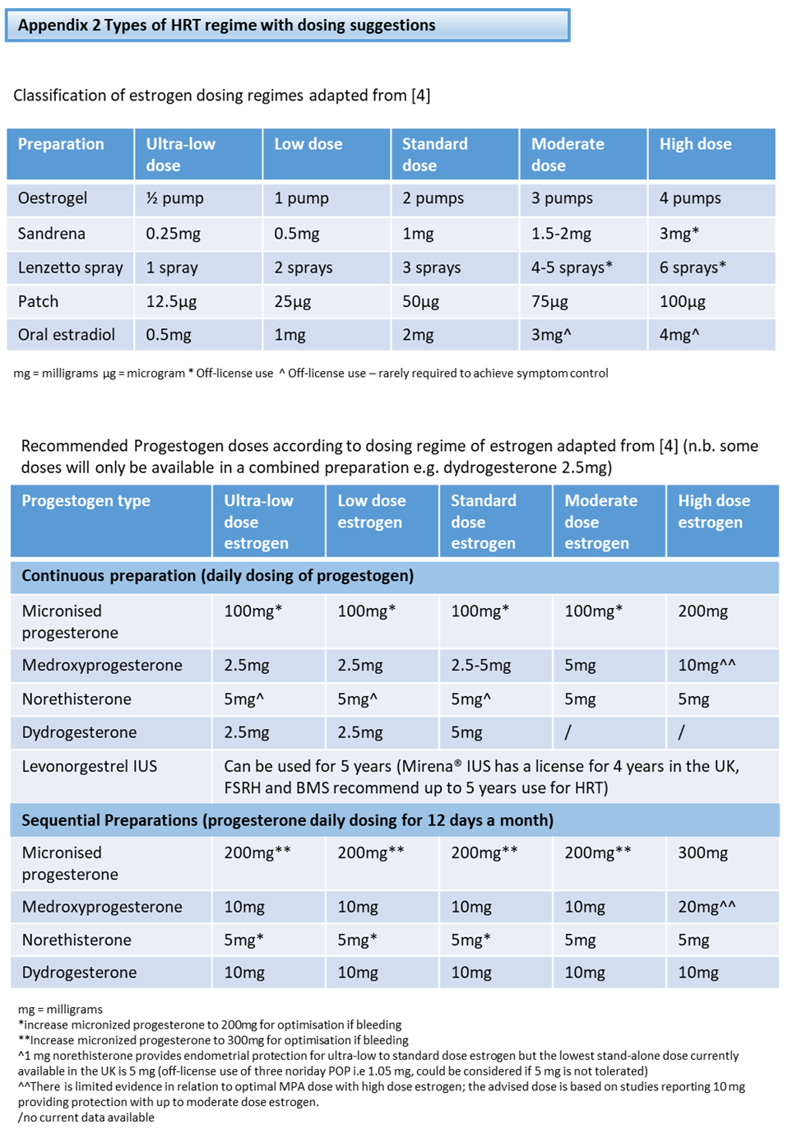
Endometrial biopsy is required if the ET exceeds 4mm (no HRT or continuous combined HRT) or exceeds 7mm (sequential HRT) [4]. The sample should be sent as ‘Urgent’ to the pathology laboratory.
Endometrial biopsy is also required if findings include:
- Incomplete or poor view of ET on ultrasound i.e the endometrial stripe is not seen along its length.
- fluid within the endometrial cavity.
- irregularity of the endometrial ECHO, regardless of the ET measurement. These women should also undergo hysteroscopy
Biopsy result and insufficient sample
If the histology report states ‘insufficient tissue for analysis’ and the ET has been thickened then the patient should have a hysteroscopy, if this hasn’t already been done, and a repeat biopsy.
Where hysteroscopy is reassuring and biopsy is insufficient, patient can be reassured and discharged from follow up. Those with recurrent symptoms after 6 months should be re-investigated.
Hysteroscopy
This provide direct visualisation of the endometrial cavity. It can also facilitate directed biopsy from concerning lesions, polyps, and polypectomy. This can be performed in the outpatient or inpatient setting.
Additional considerations
- This guideline refers to women who present with PMB for the first time.
- Those with recurrent symptoms after 6 months should be re-investigated.
- Those with persistent symptoms within 6 months should be re-referred for hysteroscopy and biopsy if not already performed (PMB no HRT)
- There is little evidence to support the use of endometrial thickness measurements and the associated cut-offs in the assessment of women with peri-menopausal bleeding without HRT. These women should undergo clinical evaluation as per menstrual disorder guideline