Synergistic Gentamicin for Endocarditis

All Patients with suspected or proven endocarditis should be discussed with microbiology.
Synergistic (low dose) gentamicin is prescribed together with a penicillin or vancomycin in the initial treatment of native valve endocarditis due to enterococcal and streptococcal species and in prosthetic valve endocarditis of all aetiology including staphylococci.
Initial Dosing:
Calculate creatinine clearance (CrCl) via MDCalc (age, height, weight and serum creatinine required) and use the table to prescribe an initial dose of gentamicin.
Prescribing:
Prescribe on the regular section of the NHS Fife drug chart; do not use the treatment gentamicin prescribing, administration, monitoring form. Doses should be administered by IV bolus injection over 3-5 minutes.
Monitoring:
For synergistic gentamicin, both peak and trough levels are measured. The aim is to produce a peak (1 hour post dose) of 3-5mg/L and a trough (immediately before a dose) of <1mg/L.
Take the first sample one hour after the first dose (peak). Take the second sample immediately before the second dose (trough).
Record the exact time taken of all gentamicin samples in the clinical details section of the electronic request. Give the second gentamicin dose whilst awaiting levels unless concerns regarding renal function. Record sampling times and levels in the patient’s medical notes.
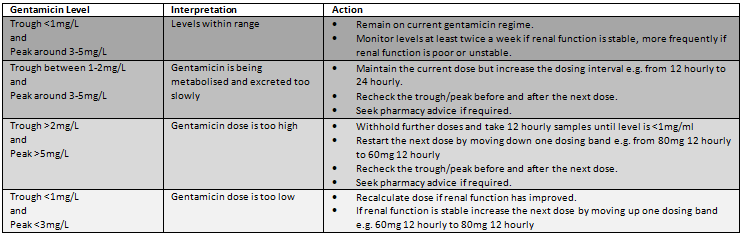
Interpretation of levels:
Monitor for toxicity:
- Renal toxicity: Monitor creatinine daily. Signs of renal toxicity include an increase of creatinine or a decrease in urine output/oliguria
- Ototoxicity: Suggested by any of the following – new tinnitus, dizziness, poor balance, hearing loss, oscillating vision, nausea or vomiting.
- Stop treatment and seek advice if toxicity develops.