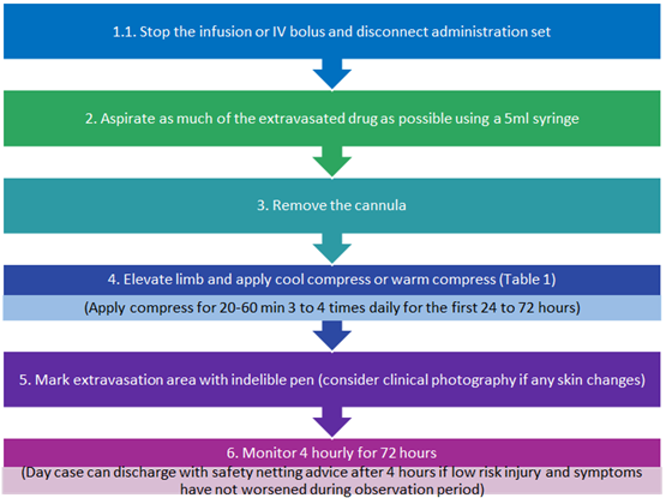
The initial management algorithm below summaries the initial actions that should occur once extravasation is detected or suspected to minimise ensuing tissue injury.

Extravasation is the inadvertent leakage of intravenous (IV) fluid or medication into extravascular tissue from a vascular access device such as a peripheral venous cannula (PVC). This guideline is for use for the management of peripheral venous extravasation not involving systemic anti-cancer therapies; please refer to separate NHS Highland Policy for the Management of Extravasation of a Systemic Anti-Cancer Therapy (SACT) including Cytotoxic Agents POL004.pdf
Extravasation has the potential to cause significant harm including pain, swelling, blistering and tissue damage and in some rare circumstances can result in compartment syndrome and even amputation. Therefore, it is vitally important that all steps are taken to minimise the risk of extravasation including daily review of PVC sites including visual infusion phlebitis score (VIP score) as per NHS Highland policy, monitoring of PVC sites during infusions and flushing PVC’s with 0.9% sodium chloride before and after administration of an IV drug. All staff should be alert to signs of possible extravasation which include increased resistance on injection, pain on injection and swelling around the cannula site.
Drugs have the potential to cause tissue damage through various pathophysiological mechanisms, including vasoconstriction and ischaemic necrosis, direct toxicity, osmotic damage and volume-related mechanical compression. The degree of tissue damage that can occur following extravasation is affected by a variety of factors including volume and type of drug administered. Some drugs have the potential to cause significant skin or tissue damage when they extravasate and these drugs are classified as vesicants (see Table 1 for examples).