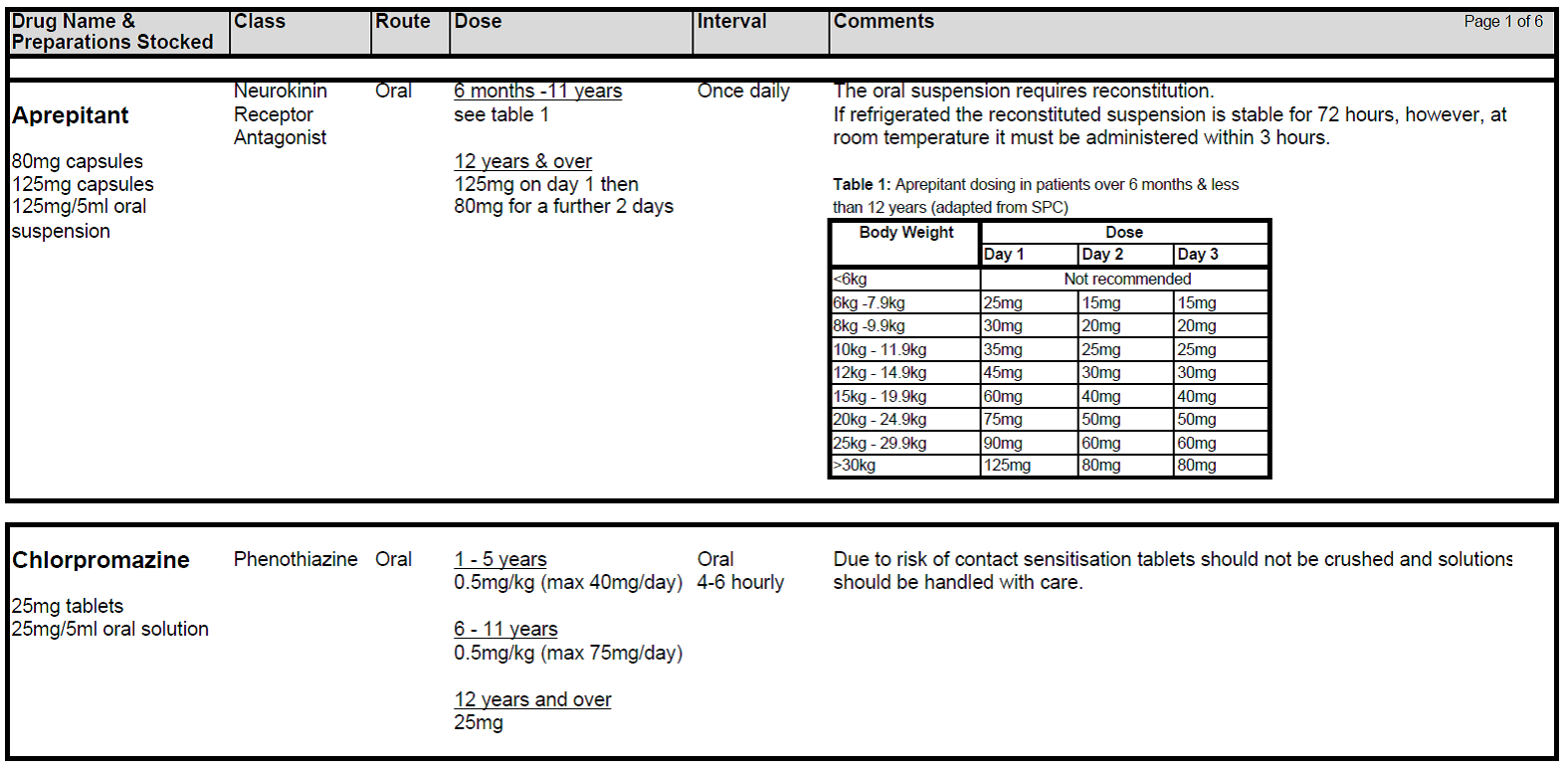
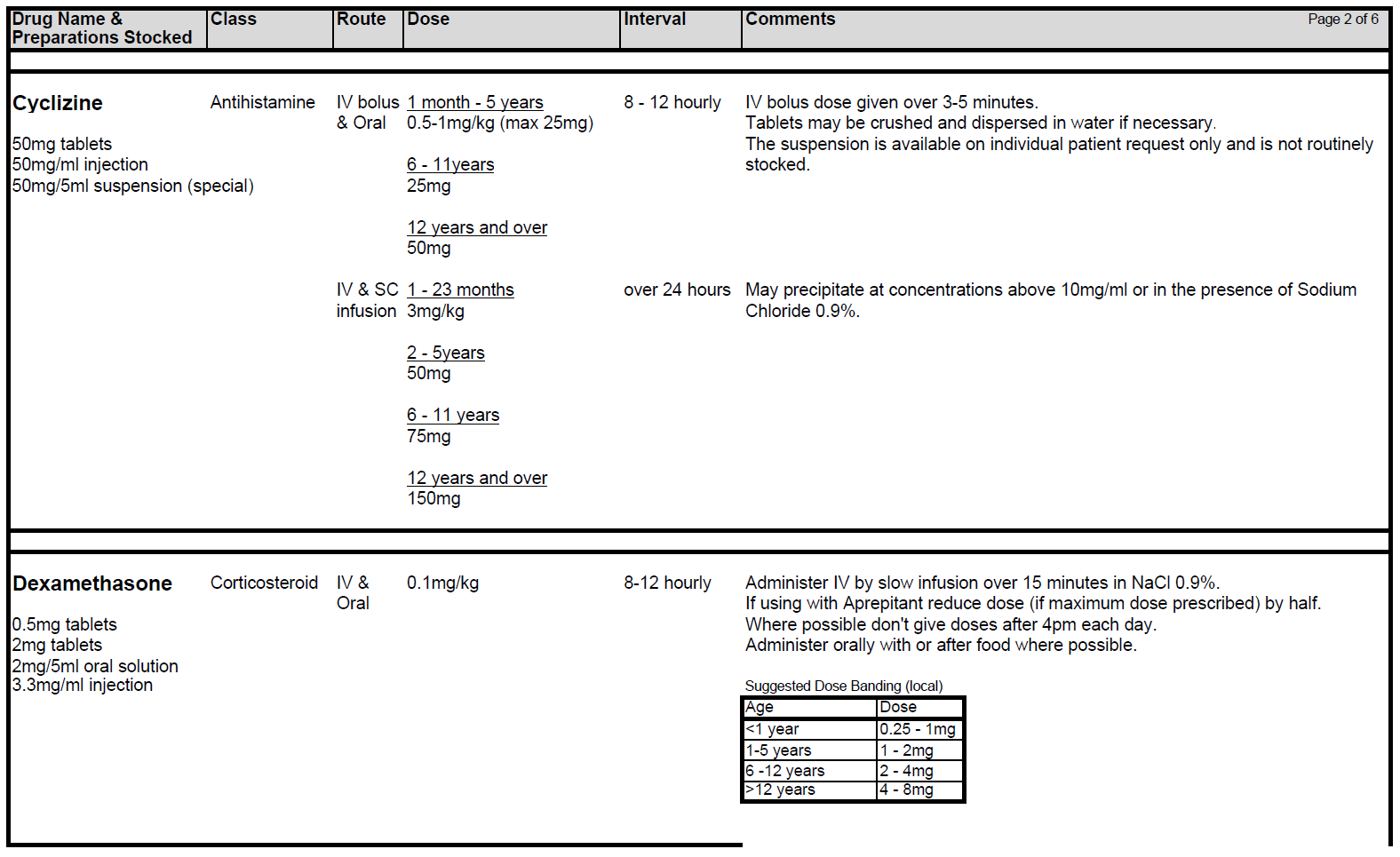
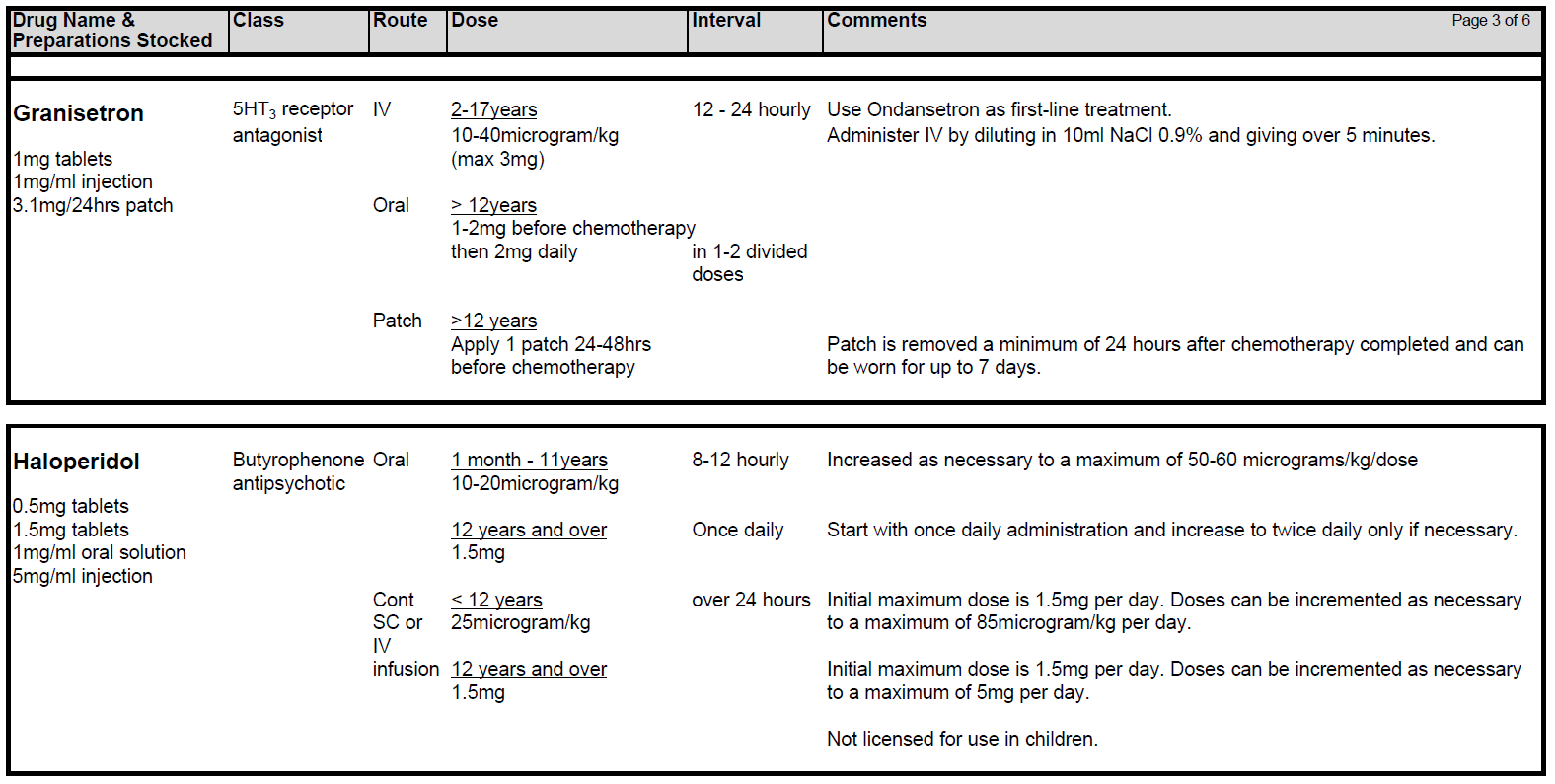
5.2.1 Patients receiving chemotherapy with a very high level of emetic risk should receive combination anti-emetic therapy with a neurokinin receptor antagonists (Aprepitant), a 5HT3 blocker (Ondansetron) and a corticosteroid (Dexamethasone). For chemotherapy with a high emetogenic potential, Ondansetron and Dexamethasone should be used as first line anti-emetic therapy, however, Aprepitant can be added to this combination as second-line therapy. Patients receiving moderately emetogenic chemotherapy will also require dual therapy with Ondansetron and Dexamethasone. Children receiving chemotherapy with a low emetic risk should receive only Ondansetron as required. If the chemotherapy agents have minimal emetogenicity, no routine prophylaxis is required. The choice of anti-emetic treatment detailed above assumes no contraindications to any of the drugs. Intravenous anti-emetics should be given at least 30 minutes prior to starting chemotherapy or orally at least 1 hr prior to starting treatment. Anti-emetics must be prescribed regularly on the appropriate patient’s prescription.
5.2.2 Choice of 5HT3 antagonist: First choice is Ondansetron. If Ondansetron is not tolerated or effective, second line should be Granisetron. If both Ondansetron and Granisetron are not tolerated or deemed ineffective, Palonosetron can be considered. Please note Palonosetron is GG&C non-formulary at present. Please discuss with the consultant and the clinical pharmacist if Palonosetron is required. There needs to be a robust clinical governance policy in place which also incorporates any shared care centres, to prevent administration of any additional 5HT3 antagonist for a minimum of 5 days post Palonosetron.
5.2.3 Dexamethasone should not be given to patients who receive steroids as part of their treatment protocol. Patients with Acute Lymphoblastic Leukaemia (ALL) receive Dexamethasone as part of induction and intensification therapy. Chemotherapy for ALL is not generally highly emetogenic and additional Dexamethasone for emesis should not be prescribed.
Patients with tumours within the Central Nervous System should not be started on Dexamethasone for as anti-emetic therapy as it may prevent chemotherapy penetrating the blood brain barrier.
Patients receiving immunomodulatory therapy should not receive steroids during that part of their treatment eg Neuroblastoma receiving Dinutuximab and Osteosarcoma receiving mifamurtide.
5.2.4 Dexamethasone should also be avoided in patients receiving conditioning treatment with chemotherapy and/or radiotherapy prior to stem cell transplantation, because steroid therapy will increase the risk of fungal infection in a population already at risk.
5.2.5 All children who face prolonged, profound neutropenia are at risk of invasive fungal infections, should avoid having Dexamethasone as antiemetic. This particularly relates to children with Acute Myeloid Leukaemia (AML) who are receiving, or have received, chemotherapy regimens containing Fludarabine and/or Gemtuzumab Ozogamicin (Mylotarg). After ensuring that Ondansetron has been prescribed at optimal dosing, failure of emetic control in these patients should be discussed with the consultant.
5.2.6 If standard anti-emetic therapy fails in any patient, exclude all other causes of nausea and/or vomiting and ensure anti-emetic drugs have been prescribed at optimal route, dosing and frequency. Check patient compliance and tolerance and consider if drug absorption might play a role in lack of response. If second line agents are considered necessary Levomepromazine is generally the drug of choice although both Metoclopramide and Cyclizine are also useful.
5.2.7 Levomepromazine (Nozinan) is a receptor non-specific agent that is often helpful in refractory cases although sedation can be problem. Beware of increased risk of extra-pyramidal side-effects and increased sedative effects when combining Metoclopramide with Cyclizine. Nabilone and Haloperidol may also be useful in certain circumstances.
5.2.8 Metoclopramide can be used in combination with Ondansetron for those receiving very highly or high emetogenic chemotherapy with a contraindication to Dexamethasone and/or Aprepitant. Metoclopramide is contra-indicated in children < 1 year. In children aged 1-18, Metoclopramide should be used as a second-line option for prevention of delayed chemotherapy-induced nausea and vomiting. Metoclopramide should only be prescribed for short-term use (<5 days). Avoid Cyclizine and Metoclopramide together – Metoclopramide is a prokinetic (stimulates the gut) while Cyclizine slows it down. They can be used together in a palliative care setting.
5.2.9 Consider using Lorazepam prior to a treatment block if either anticipatory nausea is a problem or anxiety forms a large component of the nauseating trigger (particular issue in adolescents). This may need to commence before starting the journey to hospital.
5.2.10 Remember, anti-emetics have their own side effect profile (e.g. 5HT3 blockers are constipating and can cause headache).
NB: All episodes of refractory nausea and vomiting should be discussed with a consultant.