Apixaban for the management of suspected DVT in the community

1. Drug: Apixaban 5mg Tablets.
2. NHS Borders indication: Treatment of suspected deep vein thrombosis (DVT), in
adults.
3. Background: It is not uncommon for patients with possible DVT to attend a GP surgery, distant from the BGH, late in the day. This causes difficulties with transport and often ends up with an avoidable overnight admission.
To avoid this issue it is proposed that GP’s keep a stock of Apixaban in the surgery and if, on risk assessment, clinical suspicion of a DVT is likely, and overnight anticoagulation is not contraindicated, two 10mg doses of Apixaban can be given to allow hospital attendance at AAU the next morning.
4. Risk assessment of DVT
Clinical diagnosis is notoriously unreliable. However, history and examination plus a Well’s score should be applied.
Table 1 Well’s score.
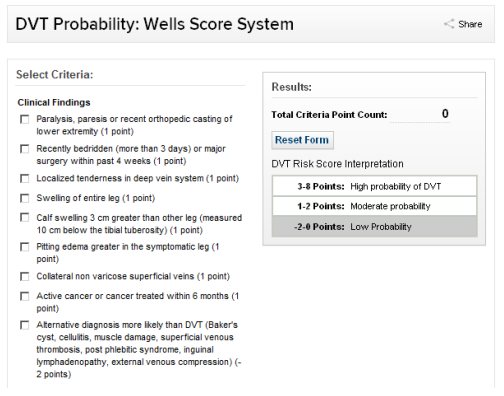

If Well’s score is 2 or more, consider whether patient can be managed as a delayed admission with Apixaban treatment at home overnight.
5. Patient selection
- Patients who have complex medical issues, massive DVT, known renal or liver failure or concomitant malignancy need to be sent to hospital immediately.
- Patients who are obviously unwell should not be treated under this protocol.
- Patients with contraindications to anticoagulation (see 6 below) should also be sent for immediate assessment.
- However, many can be given 2 doses of apixaban 10 mg to be taken pm and am (12 hours apart) with prompt attendance at AAU thereafter.
- Patients who are stable and do not have complex medical issues or contraindication to this approach can be safely anticoagulated overnight.
- Vast majority of patients with severe renal or liver disease will be known. If no bloods are available in a previously uncomplicated patient 2 doses of apixaban can be safely given as even if did have unknown renal or liver failure it will not accumulate in such a short timescale.
- PATIENTS MUST ATTEND AAU THE FOLLOWING MORNING (INCLUDING SATURDAY & SUNDAY). GP SHOULD CONTACT THE UNIT ON THE DAY OF REVIEW (VIA 1111) SO THAT STAFF ARE AWARE THAT PATIENT IS TO ATTEND. REFERRAL CAN BE 24 HOURS BUT AAU IS OPEN FROM 0800-2200.
- Apixaban does not invalidate d dimer assay so this can be used once patient attends hospital to decide whether scan is required.
6. Contraindications to apixaban
- Pregnant or breast feeding.
- Known allergy to apixaban.
- Known bleeding disorder (haemophilia, von willebrands, platelet function defect,
thrombocytopaenia). - Recent major haemorrhage ,major surgery, eye surgery (6 weeks).
- Within 6/12 CVA.
- Any history of intracerebral bleed/neurosurgery.
- If prescribed any antiplatelet (apart from aspirin) eg Clopidogrel, ticagrelor, prasugrel or already anticoagulated.
- Clinical signs of easy bleeding/bruising/petechiae massive bruising etc.
- Severe renal disease (if known to have creatinine clearance <15ml/min do not use
apixaban). - Severe liver disease with coagulopathy.
- Refer to apixaban SPC/BNF for further information.
7. Dose and supply:
Two doses of 10mg apixaban (2x 5mg tablets) to be taken 12 hours apart (GP surgeries can order prepacks of 4 x 5mg Apixaban tablets – available from BGH pharmacy - labelled with these directions).
APIXABAN IS A STANDARD TREATMENT DOSE (REGARDLESS OF WEIGHT OR AGE) OF 10 MG BD, FOR 1 WEEK THEN REDUCED TO 5MG BD.
8. Drug Interactions
- Avoid concomitant use with telithromycin.
- Not to be given with other anticoagulants.
- Avoid concomitant use with strong CY P34 or Pgp inducers; rifampificin, phenytoin, phenobarbital, carbazamazepine, St John’s wort etc.
- Co-prescription of apixaban with azole-antimycotics (eg ketoconazole, itraconazole,
voriconazole and posiconazole), HIV protease inhibitors (eg Ritonavir) is contra-indicated.
Refer to BNF & SPC for further interaction information.