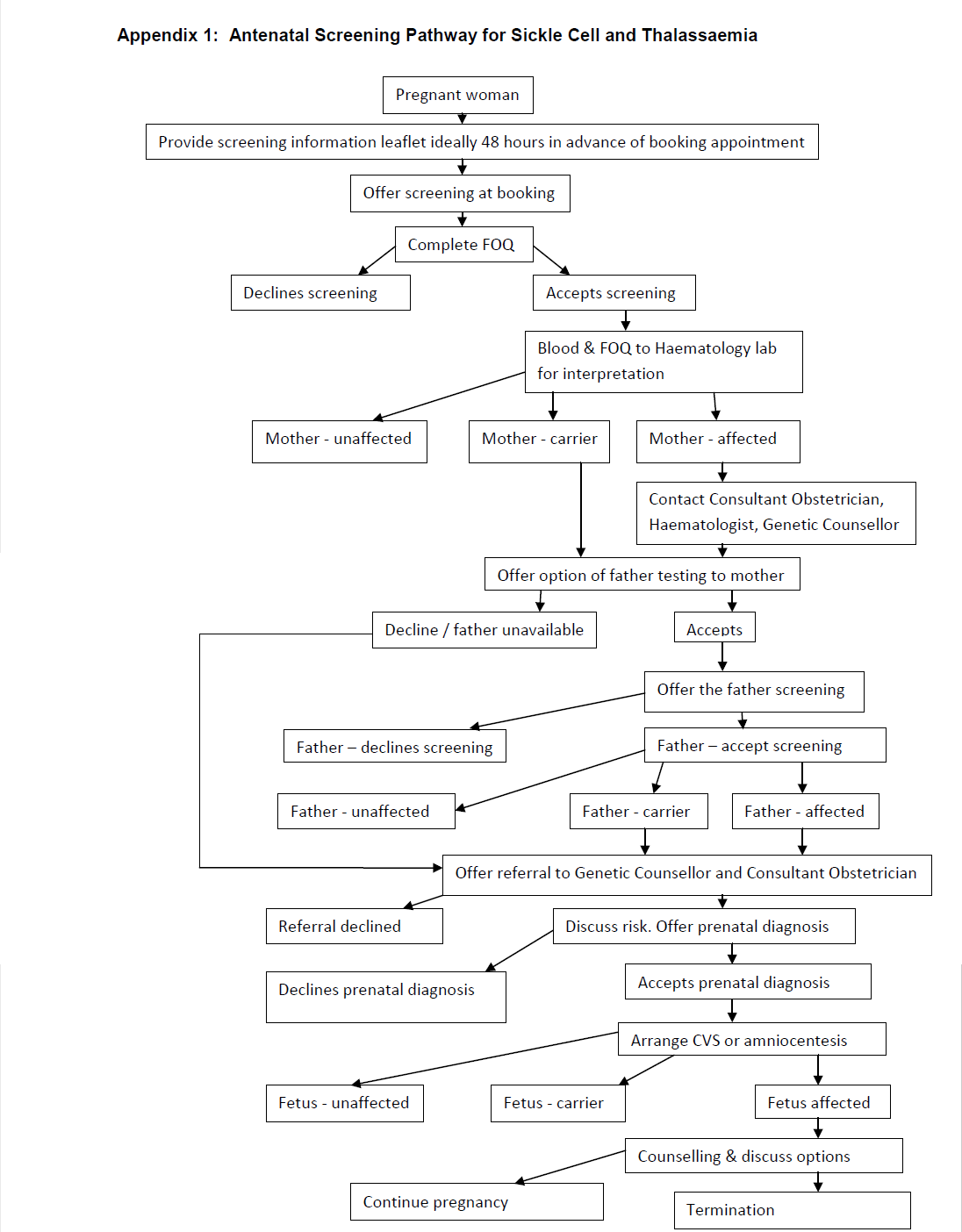
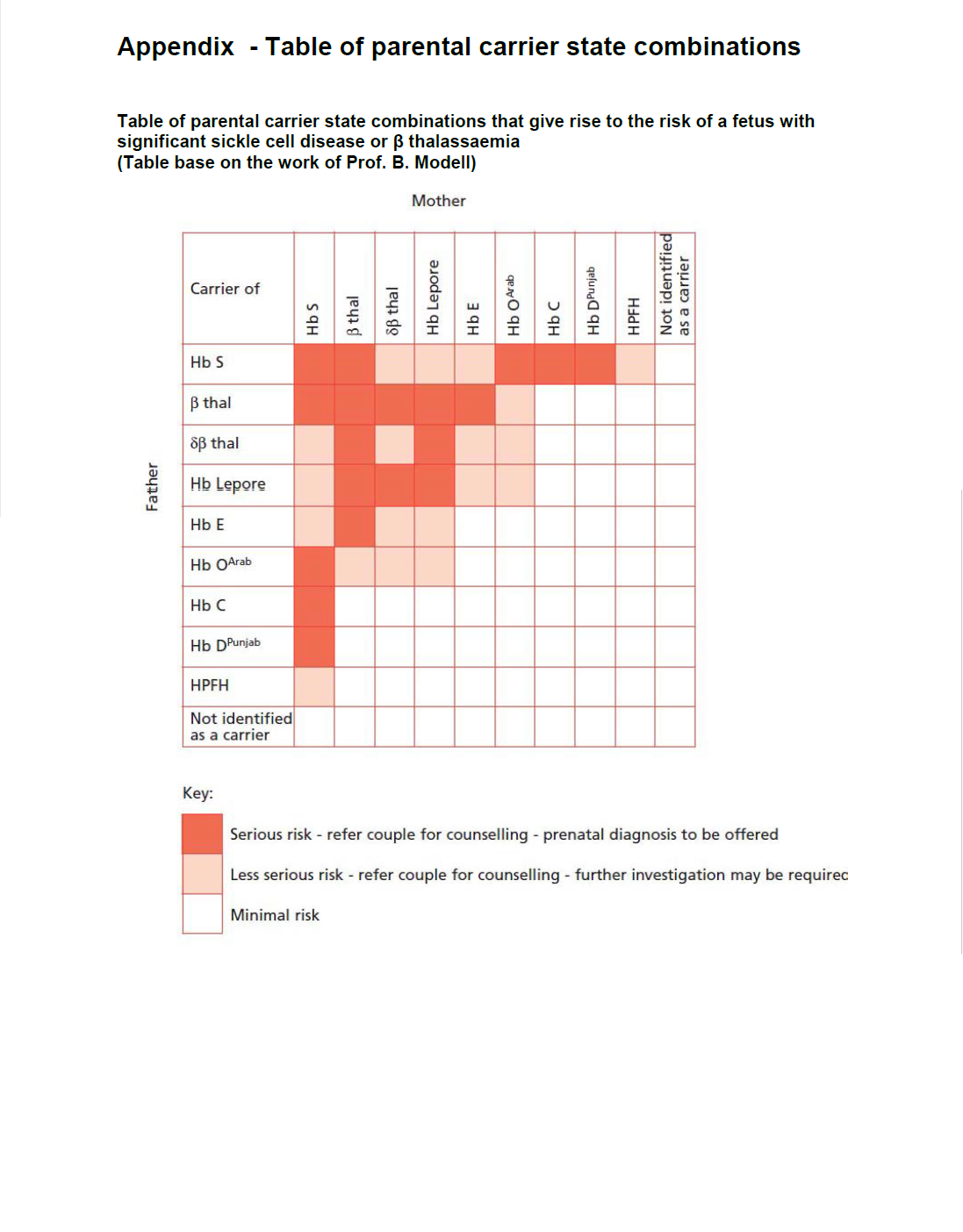
Early, ideally by 10 weeks gestation (women), to enable partners (father of baby) of screen positive women to be screened early enough to make informed choices on management. Fathers should be offered / undergone screening urgently (ideally by 12 weeks gestation). It is the responsibility of the woman’s named consultant to inform the woman of the result if she is a carrier and organise partner screening.
All women should be offered haemoglobinopathy screening regardless of their gestation, acknowledging that those being screened later in pregnancy may have fewer management options available than those booking at an earlier gestation.