VTE remains a major cause of direct maternal death in the UK (MBRRACE-UK 2021).
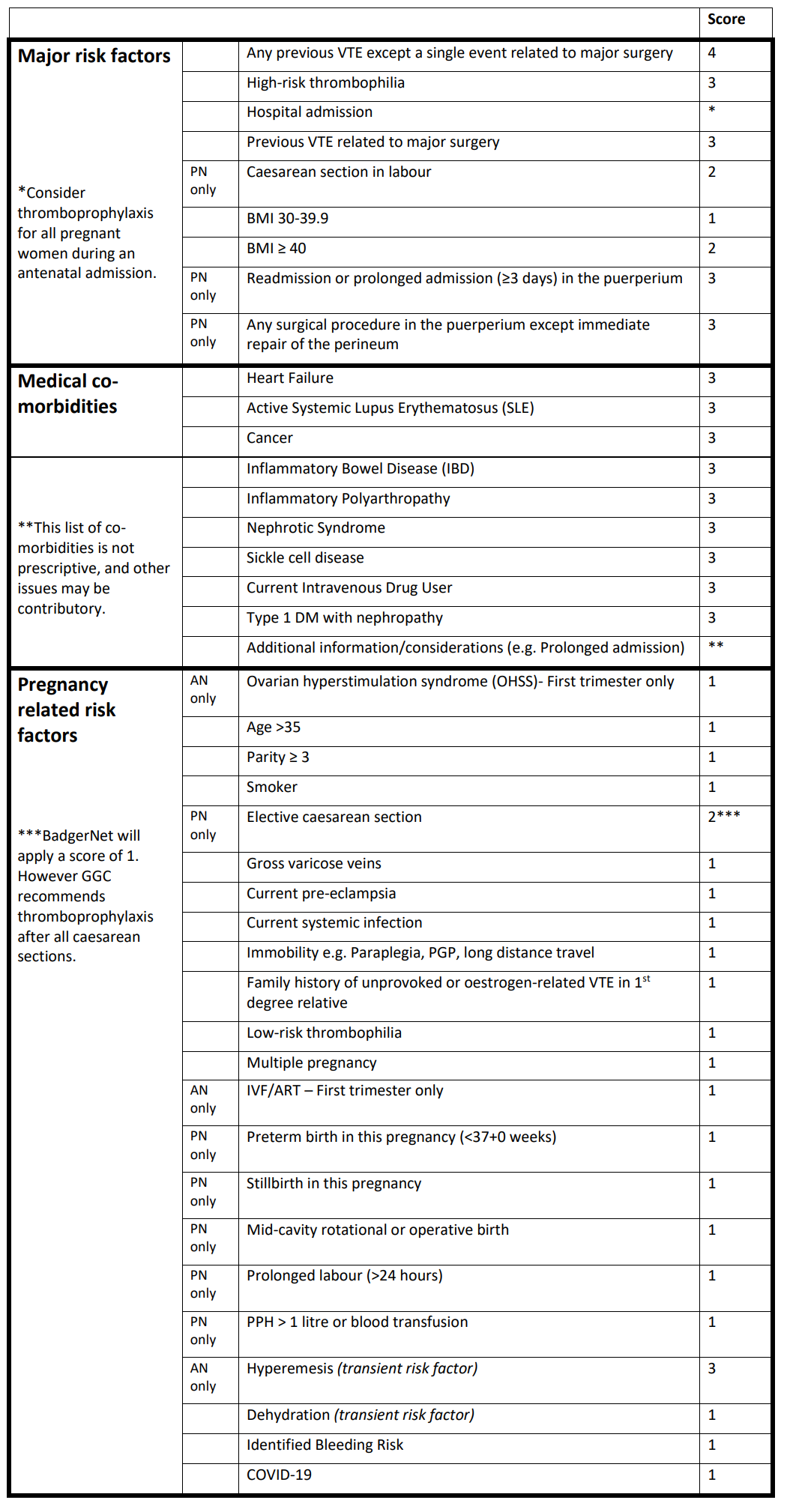
Pregnancy itself predisposes women to VTE and although the absolute risk of VTE in pregnancy is low (1-2 per 1000) [RCOG 2015], the individual likelihood of thromboembolism during pregnancy and the puerperium is influenced by a wide range of factors. The likelihood of VTE can be mitigated by offering women at increased risk thromboprophylaxis.
Factors that increase the risk include pre-existing medical conditions, or circumstances, factors specific to pregnancy and birth. Additionally some risk factors (transient risk factors) may develop and/or resolve during a pregnancy. Therefore ongoing assessment of all women from early pregnancy into the puerperium is an essential part of universal care, leading to timely identification of risk factors and provision of pharmacological prophylaxis (low molecular weight heparin – LMWH) for women at increased risk.
Infection with COVID-19 may be associated with an overall increased risk of maternal VTE. Therefore, infection should be regarded as a risk factor for VTE during pregnancy and the puerperium, and included in the risk assessment.